Durvalumab after chemoradiotherapy for locoregional recurrence of completely resected non-small-cell lung cancer (NEJ056)
- PMID: 39278260
- PMCID: PMC11531949
- DOI: 10.1111/cas.16340
Durvalumab after chemoradiotherapy for locoregional recurrence of completely resected non-small-cell lung cancer (NEJ056)
Abstract
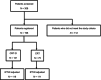
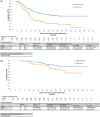
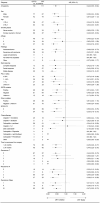
Locoregional recurrence of non-small-cell lung cancer (NSCLC) after complete resection lacks standard treatment. Durvalumab after chemoradiotherapy (CRT) or CRT alone is often selected in daily clinical practice for patients with locoregional recurrence; however, the therapeutic efficacy of these treatments remains unclear, and we aimed to assess this. This retrospective observational study used data from patients with NSCLC diagnosed with locoregional recurrence after complete resection who subsequently underwent concurrent CRT followed by durvalumab (CRT-D group) or CRT alone (CRT group). We employed propensity score analysis with inverse probability treatment weighting (IPTW) to adjust for various confounders and evaluate efficacy in the CRT-D group. After IPTW adjustment, the CRT-D group contained 119 patients (64.7% male; 69.7% adenocarcinoma), and the CRT group contained 111 patients (60.5% male; 73.4% adenocarcinoma). Their mean ages were 66 and 65 years, respectively. The IPTW-adjusted median progression-free survival was 25.4 and 11.5 months for the CRT-D and CRT groups, respectively (hazard ratio, 0.44; 95% confidence interval, 0.30-0.64); the median overall survival was not reached in either group favoring CRT-D (hazard ratio, 0.49; 95% confidence interval, 0.24-0.99). Grade 3 or 4 adverse events were observed in 48.8% of patients during CRT, 10.7% after initiating durvalumab maintenance therapy in the CRT-D group, and 57.3% in the CRT group. Overall, the sequential approach of CRT followed by durvalumab is a promising treatment strategy for locoregional recurrence of NSCLC after complete resection.
Keywords: chemoradiotherapy; durvalumab; inverse probability treatment weighting; locoregional recurrence; non–small‐cell lung cancer.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Hajime Asahina received research funding from AstraZeneca. Hiroshi Yokouchi received research funding and honoraria from AstraZeneca.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

