Suppression of neuronal AMPKβ2 isoform impairs recognition memory and synaptic plasticity
- PMID: 39278510
- PMCID: PMC11539201
- DOI: 10.1016/j.nbd.2024.106664
Suppression of neuronal AMPKβ2 isoform impairs recognition memory and synaptic plasticity
Abstract
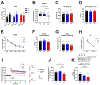
AMP-activated protein kinase (AMPK) is an αβγ heterotrimer protein kinase that functions as a molecular sensor to maintain energy homeostasis. Accumulating evidence suggests a role of AMPK signaling in the regulation of synaptic plasticity and cognitive function; however, isoform-specific roles of AMPK in the central nervous system (CNS) remain elusive. Regulation of the AMPK activities has focused on the manipulation of the α or γ subunit. Meanwhile, accumulating evidence indicates that the β subunit is critical for sensing nutrients such as fatty acids and glycogen to control AMPK activity. Here, we generated transgenic mice with conditional suppression of either AMPKβ1 or β2 in neurons and characterized potential isoform-specific roles of AMPKβ in cognitive function and underlying mechanisms. We found that AMPKβ2 (but not β1) suppression resulted in impaired recognition memory, reduced hippocampal synaptic plasticity, and altered structure of hippocampal postsynaptic densities and dendritic spines. Our study implicates a role for the AMPKβ2 isoform in the regulation of synaptic and cognitive function.
Keywords: AMPK; Isoform; LTP; Memory; Mouse model; Synaptic plasticity.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no conflicts of interest to disclose.
Figures

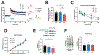

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

