SMAC mimetic drives microglia phenotype and glioblastoma immune microenvironment
- PMID: 39278921
- PMCID: PMC11402972
- DOI: 10.1038/s41419-024-07056-z
SMAC mimetic drives microglia phenotype and glioblastoma immune microenvironment
Abstract
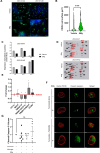
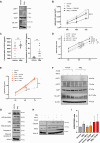
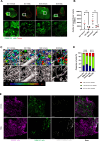
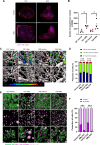
Tumor-associated macrophages/microglia (TAMs) are highly plastic and heterogeneous immune cells that can be immune-supportive or tumor-supportive depending of the microenvironment. TAMs are the most abundant immune cells in glioblastoma (GB), and play a key role in immunosuppression. Therefore, TAMs reprogramming toward immune-supportive cells is a promising strategy to overcome immunosuppression. By leveraging scRNAseq human GB databases, we identified that Inhibitor of Apoptosis Proteins (IAP) were expressed by TAMs. To investigate their role in TAMs-related immunosuppression, we antagonized IAP using the central nervous system permeant SMAC mimetic GDC-0152 (SMg). On explants and cultured immune cells isolated from human GB samples, SMg modified TAMs activity. We showed that SMg treatment promoted microglia pro-apoptotic and anti-tumoral function via caspase-3 pro-inflammatory cleavage and the inhibition of tumoroids growth. Then we designed a relevant immunogenic mouse GB model to decipher the spatio-temporal densities, distribution, phenotypes and function of TAMs with or without SMg treatment. We used 3D imaging techniques, a transgenic mouse with fluorescent TAM subsets and mass cytometry. We confirmed that SMg promoted microglia activation, antigen-presenting function and tumor infiltration. In addition, we observed a remodeling of blood vessels, a decrease in anti-inflammatory macrophages and an increased level of monocytes and their mo-DC progeny. This remodeling of the TAM landscape is associated with an increase in CD8 T cell density and activation. Altogether, these results demonstrated that SMg drives the immunosuppressive basal microglia toward an active phenotype with pro-apoptotic and anti-tumoral function and modifies the GB immune landscape. This identifies IAP as targets of choice for a potential mechanism-based therapeutic strategy and SMg as a promising molecule for this application.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

