Systematic analysis of Fc mutations designed to reduce binding to Fc-gamma receptors
- PMID: 39279104
- PMCID: PMC11407402
- DOI: 10.1080/19420862.2024.2402701
Systematic analysis of Fc mutations designed to reduce binding to Fc-gamma receptors
Erratum in
-
Correction.MAbs. 2024 Jan-Dec;16(1):2416272. doi: 10.1080/19420862.2024.2416272. Epub 2024 Oct 20. MAbs. 2024. PMID: 39428558 Free PMC article. No abstract available.
Abstract
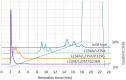
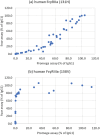
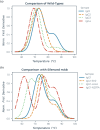
Elimination of the binding of immunoglobulin Fc to Fc gamma receptors is highly desirable for the avoidance of unwanted inflammatory responses to therapeutic antibodies and fusion proteins. Many different approaches have been used in the clinic, but they have not been systematically compared. We have now produced a matched set of anti-CD20 antibodies with different Fc subclasses and variants and compared their activity for binding to C1q, Fc-gamma receptors and in cell-based assays. Most of the variants still have significant levels of activity in one or more of these assays and many of them have impaired temperature stability compared with the corresponding wild-type antibody.
Keywords: C1q; CD16; CD32; CD64; DSF; Fc receptor; Fc region; Fc silent; FcγRI; FcγRII; FcγRIII; antibody effector function; antibody engineering; therapeutic antibody.
Conflict of interest statement
This research was sponsored by mAbsolve Limited. mAbsolve has a proprietary interest in STR mutations and licenses them to the biotech and pharmaceutical industry. Geoff Hale and Ian Wilkinson have financial interests in mAbsolve.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
