Darolutamide in Combination With Androgen-Deprivation Therapy in Patients With Metastatic Hormone-Sensitive Prostate Cancer From the Phase III ARANOTE Trial
- PMID: 39279580
- PMCID: PMC11654448
- DOI: 10.1200/JCO-24-01798
Darolutamide in Combination With Androgen-Deprivation Therapy in Patients With Metastatic Hormone-Sensitive Prostate Cancer From the Phase III ARANOTE Trial
Abstract
Purpose: For patients with metastatic hormone-sensitive prostate cancer (mHSPC), delaying progression to castration-resistant disease is important not only for overall survival (OS) but also for patients' quality of life. Darolutamide plus androgen-deprivation therapy (ADT) with docetaxel improved OS versus ADT and docetaxel in patients with mHSPC. The ARANOTE trial evaluated darolutamide and ADT without chemotherapy in patients with mHSPC.
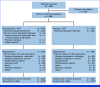
Methods: In this global phase III trial, patients were randomly assigned 2:1 to receive darolutamide 600 mg twice daily or placebo, with concomitant ADT. The primary end point was radiological progression-free survival (rPFS).
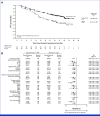
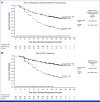
Results: From March 2021 to August 2022, 669 patients were randomly assigned (darolutamide n = 446; placebo n = 223). At the primary cutoff date (June 7, 2024), darolutamide plus ADT significantly improved rPFS, reducing the risk of radiological progression or death by 46% versus placebo plus ADT (hazard ratio [HR], 0.54 [95% CI, 0.41 to 0.71]; P < .0001), with consistent benefits across subgroups, including high- and low-volume disease. OS results were suggestive of benefit with darolutamide versus placebo (HR, 0.81 [95% CI, 0.59 to 1.12]), and clinical benefits were seen across all other secondary end points, including delayed time to metastatic castration-resistant prostate cancer (HR, 0.40 [95% CI, 0.32 to 0.51]) and time to pain progression (HR, 0.72 [95% CI, 0.54 to 0.96]). Adverse events were similar in the two groups. Notably, the incidence of fatigue was lower in patients receiving darolutamide (5.6%) versus those receiving placebo (8.1%), and fewer patients receiving darolutamide (6.1%) versus placebo (9.0%) discontinued treatment because of adverse events.
Conclusion: These results confirm the efficacy and tolerability of darolutamide plus ADT in patients with mHSPC, demonstrating clinically and statistically significant improvement in rPFS and a favorable safety profile consistent with prior phase III darolutamide trials.
Trial registration: ClinicalTrials.gov NCT04736199.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Cornford P, Tilki D, van den Bergh RCN, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Arnhem, the Netherlands: EAU Guidelines Office; 2024.
-
- Fizazi K, Chi KN. Abiraterone in metastatic prostate cancer. N Engl J Med. 2017;377:1697–1698. - PubMed
-
- Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. - PubMed
-
- James ND, Spears MR, Sydes MR. Abiraterone in metastatic prostate cancer. N Engl J Med. 2017;377:1696–1697. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

