Enteroids to Study Pediatric Intestinal Drug Transport
- PMID: 39279643
- PMCID: PMC11462498
- DOI: 10.1021/acs.molpharmaceut.4c00339
Enteroids to Study Pediatric Intestinal Drug Transport
Abstract
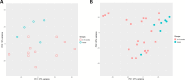
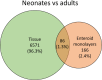
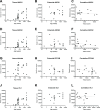
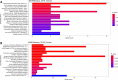
Intestinal maturational changes after birth affect the pharmacokinetics (PK) of drugs, having major implications for drug safety and efficacy. However, little is known about ontogeny-related PK patterns in the intestine. To explore the accuracy of human enteroid monolayers for studying drug transport in the pediatric intestine, we compared the drug transporter functionality and expression in enteroid monolayers and tissue from pediatrics and adults. Enteroid monolayers were cultured of 14 pediatric [median (range) age: 44 weeks (2 days-13 years)] and 5 adult donors, in which bidirectional drug transport experiments were performed. In parallel, we performed similar experiments with tissue explants in Ussing chamber using 11 pediatric [median (range) age: 54 weeks (15 weeks-10 years)] and 6 adult tissues. Enalaprilat, propranolol, talinolol, and rosuvastatin were used to test paracellular, transcellular, and transporter-mediated efflux by P-gp and breast cancer resistance protein (BCRP), respectively. In addition, we compared the expression patterns of ADME-related genes in pediatric and adult enteroid monolayers with tissues using RNA sequencing. Efflux transport by P-gp and BCRP was comparable between the enteroids and tissue. Efflux ratios (ERs) of talinolol and rosuvastatin by P-gp and BCRP, respectively, were higher in enteroid monolayers compared to Ussing chamber, likely caused by experimental differences in model setup and cellular layers present. Explorative statistics on the correlation with age showed trends of increasing ER with age for P-gp in enteroid monolayers; however, it was not significant. In the Ussing chamber setup, lower enalaprilat and propranolol transport was observed with age. Importantly, the RNA sequencing pathway analysis revealed that age-related variation in drug metabolism between neonates and adults was present in both enteroids and intestinal tissue. Age-related differences between 0 and 6 months old and adults were observed in tissue as well as in enteroid monolayers, although to a lesser extent. This study provides the first data for the further development of pediatric enteroids as an in vitro model to study age-related variation in drug transport. Overall, drug transport in enteroids was in line with data obtained from ex vivo tissue (using chamber) experiments. Additionally, pathway analysis showed similar PK-related differences between neonates and adults in both tissue and enteroid monolayers. Given the challenge to elucidate the effect of developmental changes in the pediatric age range in human tissue, intestinal enteroids derived from pediatric patients could provide a versatile experimental platform to study pediatric phenotypes.
Keywords: Ussing chamber; drug transporters; enteroids; intestinal organoids; intestine; pediatrics; pharmacokinetics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Cheung K. W. K.; van Groen B. D.; Spaans E.; van Borselen M. D.; de Bruijn A. C.; Simons-Oosterhuis Y.; Tibboel D.; Samsom J. N.; Verdijk R. M.; Smeets B.; et al. A Comprehensive Analysis of Ontogeny of Renal Drug Transporters: mRNA Analyses, Quantitative Proteomics, and Localization. Clin. Pharmacol. Ther. 2019, 106 (5), 1083–1092. 10.1002/cpt.1516. - DOI - PMC - PubMed
-
- van Groen B. D.; van de Steeg E.; Mooij M. G.; van Lipzig M. M.; de Koning B. A.; Verdijk R. M.; Wortelboer H. M.; Gaedigk R.; Bi C.; Leeder J. S.; et al. Proteomics of human liver membrane transporters: a focus on fetuses and newborn infants. Eur. J. Pharm. Sci. 2018, 124, 217–227. 10.1016/j.ejps.2018.08.042. - DOI - PubMed
-
- Mooij M. G.; Schwarz U. I.; de Koning B. A.; Leeder J. S.; Gaedigk R.; Samsom J. N.; Spaans E.; van Goudoever J. B.; Tibboel D.; Kim R. B.; et al. Ontogeny of human hepatic and intestinal transporter gene expression during childhood: age matters. Drug Metab. Dispos. 2014, 42 (8), 1268–1274. 10.1124/dmd.114.056929. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

