Chronic Activation of Tubulin Tyrosination Improves Heart Function
- PMID: 39279670
- PMCID: PMC11465905
- DOI: 10.1161/CIRCRESAHA.124.324387
Chronic Activation of Tubulin Tyrosination Improves Heart Function
Abstract
Background: Hypertrophic cardiomyopathy (HCM) is the most common cardiac genetic disorder caused by sarcomeric gene variants and associated with left ventricular hypertrophy and diastolic dysfunction. The role of the microtubule network has recently gained interest with the findings that microtubule detyrosination (dTyr-MT) is markedly elevated in heart failure. Acute reduction of dTyr-MT by inhibition of the detyrosinase (VASH [vasohibin]/SVBP [small VASH-binding protein] complex) or activation of the tyrosinase (TTL [tubulin tyrosine ligase]) markedly improved contractility and reduced stiffness in human failing cardiomyocytes and thus posed a new perspective for HCM treatment. In this study, we tested the impact of chronic tubulin tyrosination in an HCM mouse model (Mybpc3 knock-in), in human HCM cardiomyocytes, and in SVBP-deficient human engineered heart tissues (EHTs).
Methods: Adeno-associated virus serotype 9-mediated TTL transfer was applied in neonatal wild-type rodents, in 3-week-old knock-in mice, and in HCM human induced pluripotent stem cell-derived cardiomyocytes.
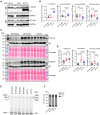
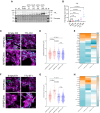
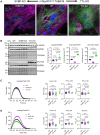
Results: We show (1) TTL for 6 weeks dose dependently reduced dTyr-MT and improved contractility without affecting cytosolic calcium transients in wild-type cardiomyocytes; (2) TTL for 12 weeks reduced the abundance of dTyr-MT in the myocardium, improved diastolic filling, compliance, cardiac output, and stroke volume in knock-in mice; (3) TTL for 10 days normalized cell area in HCM human induced pluripotent stem cell-derived cardiomyocytes; (4) TTL overexpression activated transcription of tubulins and other cytoskeleton components but did not significantly impact the proteome in knock-in mice; (5) SVBP-deficient EHTs exhibited reduced dTyr-MT levels, higher force, and faster relaxation than TTL-deficient and wild-type EHTs. RNA sequencing and mass spectrometry analysis revealed distinct enrichment of cardiomyocyte components and pathways in SVBP-deficient versus TTL-deficient EHTs.
Conclusions: This study provides the first proof of concept that chronic activation of tubulin tyrosination in HCM mice and in human EHTs improves heart function and holds promise for targeting the nonsarcomeric cytoskeleton in heart disease.
Keywords: cardiomyopathy, hypertrophic; induced pluripotent stem cells; microtubules; myocardium.
Conflict of interest statement
L. Carrier is a member of Scientific Advisory Board of and has shares in the company DiNAQOR AG (https://www.dinaqor.com/). B.L. Prosser is an inventor on a pending US patent application number 15/959181 for “Composition and Methods for Improving Heart Function and Treating Heart Failure.” The other authors report no conflicts.
Figures





Update of
-
Chronic activation of tubulin tyrosination in HCM mice and human iPSC-engineered heart tissues improves heart function.bioRxiv [Preprint]. 2024 Feb 7:2023.05.25.542365. doi: 10.1101/2023.05.25.542365. bioRxiv. 2024. Update in: Circ Res. 2024 Oct 11;135(9):910-932. doi: 10.1161/CIRCRESAHA.124.324387. PMID: 37292763 Free PMC article. Updated. Preprint.
References
-
- Carrier L. Targeting the population for gene therapy with MYBPC3. J Mol Cell Cardiol. 2021;150:101–108. doi: 10.1016/j.yjmcc.2020.10.003 - PubMed
-
- Schuldt M, Pei J, Harakalova M, Dorsch LM, Schlossarek S, Mokry M, Knol JC, Pham TV, Schelfhorst T, Piersma SR, et al. Proteomic and functional studies reveal detyrosinated tubulin as treatment target in sarcomere mutation-induced hypertrophic cardiomyopathy. Circ Heart Fail. 2021;14:e007022. doi: 10.1161/CIRCHEARTFAILURE.120.007022 - PMC - PubMed
-
- Sanyal C, Pietsch N, Ramirez Rios S, Peris L, Carrier L, Moutin MJ. The detyrosination/re-tyrosination cycle of tubulin and its role and dysfunction in neurons and cardiomyocytes. Semin Cell Dev Biol. 2023;137:46–62. doi: 10.1016/j.semcdb.2021.12.006 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

