Mechanism of Metabolic Dysfunction-associated Steatotic Liver Disease: Important role of lipid metabolism
- PMID: 39280069
- PMCID: PMC11393839
- DOI: 10.14218/JCTH.2024.00019
Mechanism of Metabolic Dysfunction-associated Steatotic Liver Disease: Important role of lipid metabolism
Abstract
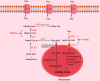
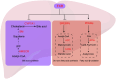
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease, has a high global prevalence and can progress to metabolic dysfunction-associated steatohepatitis, cirrhosis, and hepatocellular carcinoma. The pathogenesis of MASLD is primarily driven by disturbances in hepatic lipid metabolism, involving six key processes: increased hepatic fatty acid uptake, enhanced fatty acid synthesis, reduced oxidative degradation of fatty acids, increased cholesterol uptake, elevated cholesterol synthesis, and increased bile acid synthesis. Consequently, maintaining hepatic lipid metabolic homeostasis is essential for effective MASLD management. Numerous novel molecules and Chinese proprietary medicines have demonstrated promising therapeutic potential in treating MASLD, primarily by inhibiting lipid synthesis and promoting lipid oxidation. In this review, we summarized recent research on MASLD, elucidated the molecular mechanisms by which lipid metabolism disorders contribute to MASLD pathogenesis, and discussed various lipid metabolism-targeted therapeutic approaches for MASLD.
Keywords: Chinese proprietary medicine; Cholesterol metabolism; Lipid metabolism; Lipogenesis; Lipolysis; MASLD; lipid metabolism-targeted drugs.
© 2024 Authors.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
