Neuroprotection on ischemic brain injury by Mg2+/H2 released from endovascular Mg implant
- PMID: 39280580
- PMCID: PMC11402188
- DOI: 10.1016/j.bioactmat.2024.08.019
Neuroprotection on ischemic brain injury by Mg2+/H2 released from endovascular Mg implant
Abstract
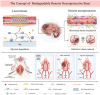
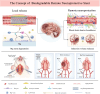
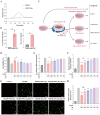
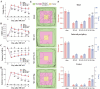
Most acute ischemic stroke patients with large vessel occlusion require stent implantation for complete recanalization. Yet, due to ischemia-reperfusion injury, over half of these patients still experience poor prognoses. Thus, neuroprotective treatment is imperative to alleviate the ischemic brain injury, and a proof-of-concept study was conducted on "biodegradable neuroprotective stent". This concept is premised on the hypothesis that locally released Mg2+/H2 from Mg metal within the bloodstream could offer synergistic neuroprotection against reperfusion injury in distant cerebral ischemic tissues. Initially, the study evaluated pure Mg's neuroactive potential using oxygen-glucose deprivation/reoxygenation (OGD/R) injured neuron cells. Subsequently, a pure Mg wire was implanted into the common carotid artery of the transient middle cerebral artery occlusion (MCAO) rat model to simulate human brain ischemia/reperfusion injury. In vitro analyses revealed that pure Mg extract aided mouse hippocampal neuronal cell (HT-22) in defending against OGD/R injury. Additionally, the protective effects of the Mg wire on behavioral abnormalities, neural injury, blood-brain barrier disruption, and cerebral blood flow reduction in MCAO rats were verified. Conclusively, Mg-based biodegradable neuroprotective implants could serve as an effective local Mg2+/H2 delivery system for treating distant cerebral ischemic diseases.
Keywords: Acute ischemic stroke; Biodegradable implantation; Hydrogen; Magnesium; Neuroprotection.
© 2024 The Authors.
Conflict of interest statement
Yufeng Zheng is the editor-in-chief for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Li C., et al. Pleiotropic microenvironment remodeling micelles for cerebral ischemia-reperfusion injury therapy by inhibiting neuronal ferroptosis and glial overactivation. ACS Nano. 2023;17:18164–18177. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

