Titanium metal-organic frameworks for photocatalytic CO2 conversion through a cycloaddition reaction
- PMID: 39280792
- PMCID: PMC11391913
- DOI: 10.1039/d4na00535j
Titanium metal-organic frameworks for photocatalytic CO2 conversion through a cycloaddition reaction
Abstract
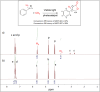
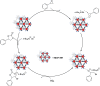
The elevated levels of CO2 in the atmosphere have been a major concern for environmental scientists. Capturing CO2 gas and its subsequent conversion to useful organic compounds is one of the avenues that have been extensively studied in the last decade. The photocatalytic cycloaddition of CO2 is a promising approach for effective CO2 capture and the production of value-added chemicals such as cyclic carbonates. MOF-901, a titanium-based metal-organic framework with hexagonal layers and imine linkages, was successfully oxidized in this study to MOF-997, incorporating amide linkages using Oxone. Both MOFs displayed remarkable photocatalytic activity in CO2 cycloaddition under mild conditions, including moderate temperatures and visible light exposure. Particularly noteworthy is MOF-997, exhibiting superior performance with donor-acceptor active sites, achieving a 99.9% yield in catalyzing CO2 conversion from styrene epoxide to styrene carbonate under solvent conditions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

