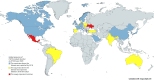
Global Presence and Penetrance of CSF1R-Related Disorder
- PMID: 39280886
- PMCID: PMC11398975
- DOI: 10.1212/NXG.0000000000200187
Global Presence and Penetrance of CSF1R-Related Disorder
Abstract
Objectives: To highlight the worldwide presence of CSF1R-related disorder (CSF1R-RD), discuss its penetrance, and provide the first haplotype analysis.
Methods: Data on patients worldwide were collected, including demographics, genotype, family history, and clinical status. For haplotype analysis, polymorphisms of short tandem repeats in 3 distinct families with CSF1R p.Ile794Thr variant were examined.
Results: Nineteen new patients were included, at a mean age of 38.7 years (ranging from 11 to 74 years), from 14 families from the Americas, Asia, Australia, and Europe, including the first from Mexico, North Macedonia, and Ukraine. Fifteen CSF1R variants were found, including 8 novel. Three patients were compound heterozygotes with disease onset at 1, 4, and 22 years. Patients with heterozygous CSF1R variants developed symptoms at a mean of 39.0 years (range 8-71 years). Four patients died at a mean of 3.3 years from onset (range 2-5 years). Negative family history was noted in 7 patients. In haplotype analysis, 2 families exhibited shared haplotype encompassing ∼6-Mb region downstream of the CSF1R while the third family displayed a different haplotype.
Discussion: CSF1R-RD has a global prevalence. The reasons for negative family history include de novo variants (as shown by the haplotype analysis), mosaicism, and incomplete penetrance, which are possibly modulated by environmental and genetic factors.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
J. Dulski is partially supported by the Haworth Family Professorship in Neurodegenerative Diseases fund (90052067). He serves as an editorial board member of Neurologia i Neurochirurgia Polska. He received speakers' bureau honoraria from VM Media Ltd., Radosław Lipiński 90 Consulting, Ipsen. He has intellectual property rights for “Application of Hydrogen Peroxide and 17β-Estradiol and its Metabolites as Biomarkers in a Method of Diagnosing Neurodegenerative Diseases In Vitro” (WO/2023/234790); R. Bruffaerts received consulting fees (advisory board) from Eisai; Z. K. Wszolek is partially supported by the NIH/NIA and NIH/NINDS (1U19AG063911, FAIN: U19AG063911), Mayo Clinic Center for Regenerative Medicine, the gifts from the Donald G. and Jodi P. Heeringa Family, the Haworth Family Professorship in Neurodegenerative Diseases fund, and The Albertson Parkinson's Research Foundation, and PPND Family Foundation. He serves as PI or Co-PI on Biohaven Pharmaceuticals, Inc. (BHV4157-206) and Vigil Neuroscience, Inc. (VGL101–01.002, VGL101–01.201, PET tracer development protocol, Csf1r biomarker and repository project, and ultra-high field MRI in the diagnosis and management of CSF1R-related adult-onset leukoencephalopathy with axonal spheroids and pigmented glia) projects/grants. He serves as Co-PI of the Mayo Clinic APDA Center for Advanced Research and as an external advisory board member for the Vigil Neuroscience, Inc., and as a consultant on neurodegenerative medical research for Eli Lilli & Company. Go to Neurology.org/NG for full disclosures.
Figures
References
-
- Bogaert V. Le type tardif de la leucodystrophie progressive familiale. Rev Neurol. 1936;65:21.
LinkOut - more resources
Full Text Sources
Miscellaneous