Bioresponsive and transformable coacervate actuated by intestinal peristalsis for targeted treatment of intestinal bleeding and inflammation
- PMID: 39280897
- PMCID: PMC11399697
- DOI: 10.1016/j.bioactmat.2024.08.020
Bioresponsive and transformable coacervate actuated by intestinal peristalsis for targeted treatment of intestinal bleeding and inflammation
Abstract
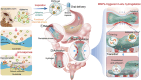
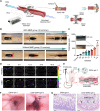
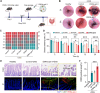
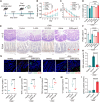
Developing an oral in situ-forming hydrogel that targets the inflamed intestine to suppress bleeding ulcers and alleviate intestinal inflammation is crucial for effectively treating ulcerative colitis (UC). Here, inspired by sandcastle worm adhesives, we proposed a water-immiscible coacervate (EMNs-gel) with a programmed coacervate-to-hydrogel transition at inflammatory sites composed of dopa-rich silk fibroin matrix containing embedded inflammation-responsive core-shell nanoparticles. Driven by intestinal peristalsis, the EMNs-gel can be actuated forward and immediately transform into a hydrogel once contacting with the inflamed intestine to yield strong tissue adhesion, resulting from matrix metalloproteinases (MMPs)-triggered release of Fe3+ from embedded nanoparticles and rearrangement of polymer network of EMNs-gel on inflamed intestine surfaces. Extensive in vitro experiments and in vivo UC models confirmed the preferential hydrogelation behavior of EMNs-gel to inflamed intestine surfaces, achieving highly effective hemostasis, and displaying an extended residence time ( 48 h). This innovative EMNs-gel provides a non-invasive solution that accurately suppresses severe bleeding and improves intestinal homeostasis in UC, showcasing great potential for clinical applications.
Keywords: Adhesive hydrogel; Bioadhesive coacervate; Bioresponsive transformation; Intestinal peristalsis drive; Intestinal ulcer bleeding.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Liu S., Cao Y., Ma L., Sun J., Ramos-Mucci L., Ma Y., Yang X., Zhu Z., Zhang J., Xiao B. Oral antimicrobial peptide-EGCG nanomedicines for synergistic treatment of ulcerative colitis. J. Contr. Release. 2022;347:544–560. - PubMed
-
- Olen O., Erichsen R., Sachs M.C., Pedersen L., Halfvarson J., Askling J., Ekbom A., Sorensen H.T., Ludvigsson J.F. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395(10218):123–131. - PubMed
LinkOut - more resources
Full Text Sources

