Suspension electrospinning of decellularized extracellular matrix: A new method to preserve bioactivity
- PMID: 39280898
- PMCID: PMC11401211
- DOI: 10.1016/j.bioactmat.2024.08.012
Suspension electrospinning of decellularized extracellular matrix: A new method to preserve bioactivity
Abstract
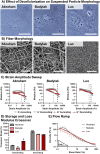
Decellularized extracellular matrices (dECM) have strong regenerative potential as tissue engineering scaffolds; however, current clinical options for dECM scaffolds are limited to freeze-drying its native form into sheets. Electrospinning is a versatile scaffold fabrication technique that allows control of macro- and microarchitecture. It remains challenging to electrospin dECM, which has led researchers to either blend it with synthetic materials or use enzymatic digestion to fully solubilize the dECM. Both strategies reduce the innate bioactivity of dECM and limit its regenerative potential. Herein, we developed a new suspension electrospinning method to fabricate a pure dECM fibrous mesh that retains its innate bioactivity. Systematic investigation of suspension parameters was used to identify critical rheological properties required to instill "spinnability," including homogenization, concentration, and particle size. Homogenization enhanced particle interaction to impart the requisite elastic behavior to withstand electrostatic drawing without breaking. A direct correlation between concentration and viscosity was observed that altered fiber morphology; whereas, particle size had minimal impact on suspension properties and fiber morphology. The versatility of this new method was demonstrated by electrospinning dECM with three common decellularization techniques (Abraham, Badylak, Luo) and tissue sources (intestinal submucosa, heart, skin). Bioactivity retention after electrospinning was confirmed using cell proliferation, angiogenesis, and macrophage polarization assays. Collectively, these findings provide a framework for researchers to electrospin dECM for diverse tissue engineering applications.
Keywords: Biological scaffolds; Electrospinning; Extracellular matrix.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Badylak S.F. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007/09/01/2007;28(25):3587–3593. - PubMed
-
- Badylak S.F., Freytes D.O., Gilbert T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009/01/01/2009;5(1):1–13. - PubMed
-
- Li F., Li W., Johnson S., Ingram D., Yoder M., Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. May-Aug 2004;11(3–4):199–206. (in eng) - PubMed
LinkOut - more resources
Full Text Sources

