Synergistic effect of ex-vivo quality and quantity cultured mononuclear cells and mesenchymal stem cell therapy in ischemic hind limb model mice
- PMID: 39281108
- PMCID: PMC11401098
- DOI: 10.1016/j.reth.2024.08.013
Synergistic effect of ex-vivo quality and quantity cultured mononuclear cells and mesenchymal stem cell therapy in ischemic hind limb model mice
Abstract
Introduction: Chronic limb-threatening ischemia (CLTI) is a condition characterized by peripheral arterial disease and tissue damage caused by reduced blood flow. New therapies using various cell types, such as mesenchymal stem cells (MSCs) and mononuclear cells (MNCs), have been developed for the patients unresponsive to conventional therapies. MSCs are promising because of their ability to secrete growth factors essential for vascularization, whereas MNCs contain endothelial progenitor cells that are important for blood vessel formation. However, conventional methods for isolating these cells have limitations, especially in patients with diabetes with dysfunctional cells. To overcome this problem, a culture method called quality and quantity cultured peripheral blood MNCs (MNC-QQ) was developed to efficiently produce high-quality cells from small amounts of peripheral blood. Combining MSCs with MNC-QQs has been hypothesized to enhance therapeutic outcomes. This study aimed to examine the angiogenic efficacy of MSCs with MNC-QQs in models with severe lower limb ischemia.
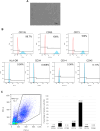
Methods: MNC-QQ was manufactured from the peripheral blood of healthy volunteers, while human bone marrow derived MSCs were purchased. To verify the effects of the MSC and MNC-QQs combination in angiogenesis, we conducted the HUVEC tube formation assay. For in vivo experiments, we created an ischemic limb model using BALB/c nude mice. Saline, MSCs alone, and a combination of MSCs and MNC-QQs were administered intramuscularly into the ischemic limbs. Blood flow was measured over time using laser doppler, and the ischemic limbs were harvested 21 days later for HE staining and immunostaining for histological assessment.
Results: In-vitro studies demonstrated increased angiogenesis when MSCs were combined with MNC-QQs compared with MSCs alone. In vivo experiments using a mouse model of severe lower limb ischemia showed that combination therapy significantly improved blood flow recovery and limb salvage compared with MSCs alone or saline treatment. Histological analysis revealed enhanced vessel density, arteriogenesis, muscle regeneration, and reduced fibrosis in the MSC + MNC-QQ group compared with those in the saline group. Although the specific interactions between MSCs and MNC-QQs have not been fully elucidated, combined therapy leverages the benefits of both cell types, resulting in improved outcomes for vascular regeneration.
Conclusions: This study highlights the potential of the simultaneous transplantation of MSCs and MNC-QQs as a promising therapeutic approach for CLTI, offering sustained long-term benefits for patients.
Keywords: Cell therapy; Mesenchymal stem cells; Peripheral blood mononuclear cell; Vasculogenesis.
© 2024 The Author(s).
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
LinkOut - more resources
Full Text Sources

