SARS-CoV-2 seroprevalence in people living with HIV in South Sudan
- PMID: 39281194
- PMCID: PMC11399597
- DOI: 10.1016/j.ijregi.2024.100421
SARS-CoV-2 seroprevalence in people living with HIV in South Sudan
Abstract
Objectives: The burden of SARS-CoV-2 infection in people living with HIV (PLHIV) in South Sudan is unknown.
Methods: We conducted a cross-sectional seroprevalence survey of SARS-CoV-2 immunoglobulin (Ig) G antibodies and other diseases of public health importance (strongyloidiasis, toxoplasmosis) in PLHIV in South Sudan during April 1, 2020-April 30, 2022. We used a multiplex SARS-CoV-2 immunoassay to detect IgG antibodies targeting the SARS-CoV-2 spike, receptor binding domain, and nucelocapsid (N) proteins, and antigens for other pathogens (Strongyloides stercoralis and Toxoplasma gondii).
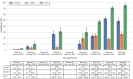
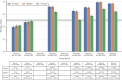
Results: Among 3518 samples tested, seroprevalence of IgG antibodies to SARS-CoV-2 spike protein and receptor binding domain 591 and nucleocapsid ranged from 1.4% (95% confidence interval [CI]: 0.9-2.1%) in April-June 2020 to 53.3% (95% CI: 49.5-57.1%) in January-March 2022. The prevalence of S. stercoralis IgG ranged between 27.3% (95% CI: 23.4-31.5%) in October-December 2021 and 47.2% (95% CI: 37.8-56.8%) in July-September 2021, and, for T. gondii IgG, prevalence ranged from 15.5% (95% CI: 13.3-17.9%) in April-June 2020 to 36.2% (95% CI: 27.4-46.2%) July-September 2021.
Conclusions: By early 2022, PLHIV in South Sudan had high rates of SARS-CoV-2 seropositivity. Surveillance of diseases of global health concern in PLHIV is crucial to estimate population-level exposure and inform public health responses.
Keywords: COVID-19; People living with HIV; SARS-CoV-2; Seroprevalence.
© 2024 The Authors.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
References
-
- World Health Organization. Weekly epidemiological update on COVID-19, https://www.who.int/publications/m/item/weekly-epidemiological-update-on...; 2022 [accessed 15 May 2022].
-
- Basavaraju SV, Patton ME, Grimm K, Rasheed MAU, Lester S, Mills L, et al. Serologic testing of US blood donations to identify severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–reactive antibodies: December 2019–January 2020. Clin Infect Dis. 2021;72 doi: 10.1093/cid/ciaa1785. e1004–9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

