Morphologic characterization and cytokine response of chicken bone-marrow derived dendritic cells to infection with high and low pathogenic avian influenza virus
- PMID: 39281683
- PMCID: PMC11401072
- DOI: 10.3389/fimmu.2024.1374838
Morphologic characterization and cytokine response of chicken bone-marrow derived dendritic cells to infection with high and low pathogenic avian influenza virus
Abstract
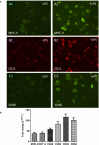
Dendritic cells (DCs) are professional antigen-presenting cells, which are key components of the immune system and involved in early immune responses. DCs are specialized in capturing, processing, and presenting antigens to facilitate immune interactions. Chickens infected with avian influenza virus (AIV) demonstrate a wide range of clinical symptoms, based on pathogenicity of the virus. Low pathogenic avian influenza (LPAI) viruses typically induce mild clinical signs, whereas high pathogenic avian influenza (HPAI) induce more severe disease, which can lead to death. For this study, chicken bone marrow-derived DC (ckBM-DC)s were produced and infected with high and low pathogenic avian influenza viruses of H5N2 or H7N3 subtypes to characterize innate immune responses, study effect on cell morphologies, and evaluate virus replication. A strong proinflammatory response was observed at 8 hours post infection, via upregulation of chicken interleukin-1β and stimulation of the interferon response pathway. Microscopically, the DCs underwent morphological changes from classic elongated dendrites to a more general rounded shape that eventually led to cell death with the presence of scattered cellular debris. Differences in onset of morphologic changes were observed between H5 and H7 subtypes. Increases in viral titers demonstrated that both HPAI and LPAI are capable of infecting and replicating in DCs. The increase in activation of infected DCs may be indicative of a dysregulated immune response typically seen with HPAI infections.
Keywords: avian influenza; chicken; cytokines; dendritic cells; innate immunity; interferon.
Copyright © 2024 Mo, Segovia, Chrzastek, Briggs and Kapczynski.
Conflict of interest statement
Author KS was employed by company CSL Seqirus. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- 2022-2023 confirmations of highly pathogenic avian influenza in commercial and backyard flocks. Avian Influenza, Riverdale Park, MD, USA: (2023). Available at: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-in....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

