This is a preprint.
Neuroimaging Biomarkers in Addiction
- PMID: 39281741
- PMCID: PMC11398440
- DOI: 10.1101/2024.09.02.24312084
Neuroimaging Biomarkers in Addiction
Abstract
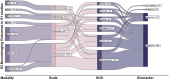
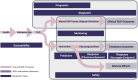
As a neurobiological process, addiction involves pathological patterns of engagement with substances and a range of behaviors with a chronic and relapsing course. Neuroimaging technologies assess brain activity, structure, physiology, and metabolism at scales ranging from neurotransmitter receptors to large-scale brain networks, providing unique windows into the core neural processes implicated in substance use disorders. Identified aberrations in the neural substrates of reward and salience processing, response inhibition, interoception, and executive functions with neuroimaging can inform the development of pharmacological, neuromodulatory, and psychotherapeutic interventions to modulate the disordered neurobiology. Based on our systematic search, 409 protocols registered on ClinicalTrials.gov include the use of one or more neuroimaging paradigms as an outcome measure in addiction, with the majority (N=268) employing functional magnetic resonance imaging (fMRI), followed by positron emission tomography (PET) (N=71), electroencephalography (EEG) (N=50), structural magnetic resonance imaging (MRI) (N=35) and magnetic resonance spectroscopy (MRS) (N=35). Furthermore, in a PubMed systematic review, we identified 61 meta-analyses including 30 fMRI, 22 structural MRI, 8 EEG, 7 PET, and 3 MRS meta-analyses suggesting potential biomarkers in addictions. These studies can facilitate the development of a range of biomarkers that may prove useful in the arsenal of addiction treatments in the coming years. There is evidence that these markers of large-scale brain structure and activity may indicate vulnerability or separate disease subtypes, predict response to treatment, or provide objective measures of treatment response or recovery. Neuroimaging biomarkers can also suggest novel targets for interventions. Closed or open loop interventions can integrate these biomarkers with neuromodulation in real-time or offline to personalize stimulation parameters and deliver the precise intervention. This review provides an overview of neuroimaging modalities in addiction, potential neuroimaging biomarkers, and their physiologic and clinical relevance. Future directions and challenges in bringing these putative biomarkers from the bench to the bedside are also discussed.
Conflict of interest statement
O.C. has received grant funding from Eli Lilly, Inc, and Nestle, Inc. He has provided paid consulting to Novo Nordisk. Dr. Paulus is an advisor to Spring Care, Inc., a behavioral health startup, he has received royalties for an article about methamphetamine in UpToDate. M.P.P. has a consulting agreement with and receives compensation from F. Hoffmann-La Roche Ltd. P.O. is an employee and shareholder of Sage Therapeutics. Other authors report no conflicts of interest. Disclaimer: The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Figures





References
-
- Degenhardt L, Charlson F, Ferrari A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Psychiatry. 2018;5(12):987–1012. doi:10.1016/S2215-0366(18)30337-7 - DOI - PMC - PubMed
-
- Shield KD, Imtiaz S, Probst C, Rehm J. The epidemiology and public health burden of addictive disorders. In: Integrating Psychological and Pharmacological Treatments for Addictive Disorders: An Evidence-Based Guide. 2018:3–31.
-
- National Institutes of Health. National Institute on Drug Abuse. Trends and Statistics. Published online 2020. Accessed May 20, 2020. https://www.drugabuse.gov/related-topics/trends-statistics/
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition.; 2013. Accessed September 8, 2020. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 - DOI
Publication types
Grants and funding
- P20 GM121312/GM/NIGMS NIH HHS/United States
- R01 AG077000/AG/NIA NIH HHS/United States
- R01 AG067765/AG/NIA NIH HHS/United States
- R01 AG062309/AG/NIA NIH HHS/United States
- U01 DA048517/DA/NIDA NIH HHS/United States
- R01 AG077497/AG/NIA NIH HHS/United States
- R01 AG041200/AG/NIA NIH HHS/United States
- RF1 AG041200/AG/NIA NIH HHS/United States
- U01 DA050989/DA/NIDA NIH HHS/United States
- T32 DA028874/DA/NIDA NIH HHS/United States
- R01 AG069476/AG/NIA NIH HHS/United States
- UG1 DA050209/DA/NIDA NIH HHS/United States
- P30 DA046345/DA/NIDA NIH HHS/United States
- R01 AG062200/AG/NIA NIH HHS/United States
- R01 DA039215/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
