A predictive machine-learning model for clinical decision-making in washed microbiota transplantation on ulcerative colitis
- PMID: 39281978
- PMCID: PMC11399476
- DOI: 10.1016/j.csbj.2024.08.021
A predictive machine-learning model for clinical decision-making in washed microbiota transplantation on ulcerative colitis
Abstract
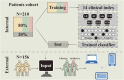
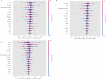
Machine learning based on clinical data and treatment protocols for better clinical decision-making is a current research hotspot. This study aimed to build a machine learning model on washed microbiota transplantation (WMT) for ulcerative colitis (UC), providing patients and clinicians with a new evaluation system to optimize clinical decision-making. Methods Patients with UC who underwent WMT via mid-gut or colonic delivery route at an affiliated hospital of Nanjing Medical University from April 2013 to June 2022 were recruited. Model ensembles based on the clinical indicators were constructed by machine-learning to predict the clinical response of WMT after one month. Results A total of 366 patients were enrolled in this study, with 210 patients allocated for training and internal validation, and 156 patients for external validation. The low level of indirect bilirubin, activated antithrombin III, defecation frequency and cholinesterase and the elderly and high level of creatine kinase, HCO3 - and thrombin time were related to the clinical response of WMT at one month. Besides, the voting ensembles exhibited an area under curve (AUC) of 0.769 ± 0.019 [accuracy, 0.754; F1-score, 0.845] in the internal validation; the AUC of the external validation was 0.614 ± 0.017 [accuracy, 0.801; F1-score, 0.887]. Additionally, the model was available at https://wmtpredict.streamlit.app. Conclusions This study pioneered the development of a machine learning model to predict the one-month clinical response of WMT on UC. The findings demonstrate the potential value of machine learning applications in the field of WMT, opening new avenues for personalized treatment strategies in gastrointestinal disorders. Trial registration clinical trials, NCT01790061. Registered 09 February 2013 - Retrospectively registered, https://clinicaltrials.gov/study/NCT01790061.
Keywords: Clinical indicator; Fecal microbiota transplant; Machine learning; Ulcerative colitis.
© 2024 The Authors.
Conflict of interest statement
Faming Zhang conceived the concept of GenFMTer and transendoscopic tubing and the devices (FMT Medical, Nanjing, China) related to them.
Figures



References
-
- Feuerstein J.D., Cheifetz A.S. Ulcerative colitis: epidemiology, diagnosis, and management [J. Mayo Clin Proc. 2014;89(11):1553–1563. - PubMed
-
- Machiels K., Joossens M., Sabino J., et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–1283. - PubMed
-
- Surawicz C.M., Brandt L.J., Binion D.G., et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. quiz 99. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
