Bursting gamma oscillations in neural mass models
- PMID: 39281982
- PMCID: PMC11392745
- DOI: 10.3389/fncom.2024.1422159
Bursting gamma oscillations in neural mass models
Abstract
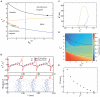
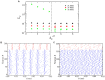
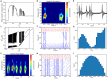
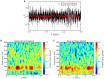
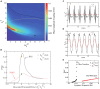
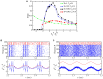
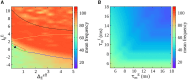
Gamma oscillations (30-120 Hz) in the brain are not periodic cycles, but they typically appear in short-time windows, often called oscillatory bursts. While the origin of this bursting phenomenon is still unclear, some recent studies hypothesize its origin in the external or endogenous noise of neural networks. We demonstrate that an exact neural mass model of excitatory and inhibitory quadratic-integrate and fire-spiking neurons theoretically predicts the emergence of a different regime of intrinsic bursting gamma (IBG) oscillations without any noise source, a phenomenon due to collective chaos. This regime is indeed observed in the direct simulation of spiking neurons, characterized by highly irregular spiking activity. IBG oscillations are distinguished by higher phase-amplitude coupling to slower theta oscillations concerning noise-induced bursting oscillations, thus indicating an increased capacity for information transfer between brain regions. We demonstrate that this phenomenon is present in both globally coupled and sparse networks of spiking neurons. These results propose a new mechanism for gamma oscillatory activity, suggesting deterministic collective chaos as a good candidate for the origin of gamma bursts.
Keywords: bursting; gamma oscillations; neural mass model; phase amplitude coupling; spiking neural network (SNN); synchronization.
Copyright © 2024 Nandi, Valla and di Volo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Benettin G., Galgani L., Giorgilli A., Strelcyn J.-M. (1980). Lyapunov characteristic exponents for smooth dynamical systems and for hamiltonian systems; a method for computing all of them. Part 1: theory. Meccanica 15, 9–20. 10.1007/BF02128236 - DOI
LinkOut - more resources
Full Text Sources

