Cellular and humoral response to SARS-CoV-2 vaccine BNT162b2 in adults with Chronic Kidney Disease G4/5
- PMID: 39282145
- PMCID: PMC11400989
- DOI: 10.1016/j.nmni.2024.101458
Cellular and humoral response to SARS-CoV-2 vaccine BNT162b2 in adults with Chronic Kidney Disease G4/5
Abstract
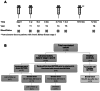
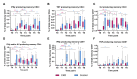
The mRNA vaccines have proven to be very effective in preventing severe disease and death from SARS-CoV-2 in the general population. However, in patients with chronic kidney disease (CKD) in dialysis or with kidney transplants (KT) the vaccine responses vary, with severe breakthrough infections as a consequence. In this intervention study we investigated the magnitude and quality of the responses to mRNA vaccination administered prior to kidney replacement therapy (KRT). Twenty patients with CKD G4/5 and nine healthy controls were followed for 12 months after receiving two doses of BNT162b2 four weeks apart and a booster dose after 3-6 months. Induction of anti-Spike and anti-RBD IgG in plasma followed the same kinetics in CKD patients and controls, with a trend towards higher titers in controls. In accordance, there was no differences in the establishment of Spike-specific memory B-cells between groups. In contrast, the CKD patients showed lower levels of anti-Spike IgG in saliva and Spike-specific CD8+ T-cells in blood, possibly influencing the capacity of viral clearance which can contribute to an elevated risk of severe breakthrough infections. In conclusion, we found that CKD patients, despite having a reduced mucosal and cytotoxic immunity to BNT162b2, demonstrated a serological response in plasma similar to healthy controls. This suggests that immunization prior to RRT is efficient and motivated. (EudraCT-nr 2021-000988-68).
Keywords: COVID-19; Chronic kidney disease; Kidney failure; SARS-CoV-2; Vaccine.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Anja Rosdahl reports financial support was provided by Orebro Research Council. Fredrika Hellgren reports travel was provided by the Karolinska Institute Foundation for Virus Research, the Karolinska Institute Travle Grant, The Swedisch Society for Immunology and the Keystone Symposia. Karin Lore reports financial support was provided by 10.13039/501100004359Swedish Research Council and 10.13039/501100002794Swedish Cancer Society. Anja Rosdahl and Helena Hervius Askling reports a relationship with National Swedish Infectious Diseases Medical Doctors Vaccine Group that includes: board membership. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Kinetics and Persistence of the Cellular and Humoral Immune Responses to BNT162b2 mRNA Vaccine in SARS-CoV-2-Naive and -Experienced Subjects: Impact of Booster Dose and Breakthrough Infections.Front Immunol. 2022 May 31;13:863554. doi: 10.3389/fimmu.2022.863554. eCollection 2022. Front Immunol. 2022. PMID: 35711445 Free PMC article.
-
Assessment of humoral and cellular immune responses to SARS CoV-2 vaccination (BNT162b2) in immunocompromised renal allograft recipients.Transpl Infect Dis. 2022 Apr;24(2):e13813. doi: 10.1111/tid.13813. Epub 2022 Mar 3. Transpl Infect Dis. 2022. PMID: 35202497 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients.Am J Kidney Dis. 2021 Oct;78(4):571-581. doi: 10.1053/j.ajkd.2021.06.002. Epub 2021 Jun 24. Am J Kidney Dis. 2021. PMID: 34174364 Free PMC article.
-
Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection.Front Immunol. 2023 Mar 7;14:1131229. doi: 10.3389/fimmu.2023.1131229. eCollection 2023. Front Immunol. 2023. PMID: 36960070 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
