This is a preprint.
Early brain neuroinflammatory and metabolic changes identified by dual tracer microPET imaging in mice with acute liver injury
- PMID: 39282308
- PMCID: PMC11398324
- DOI: 10.1101/2024.09.02.610840
Early brain neuroinflammatory and metabolic changes identified by dual tracer microPET imaging in mice with acute liver injury
Abstract
Background: Acute liver injury (ALI) that progresses into acute liver failure (ALF) is a life-threatening condition with an increasing incidence and associated costs. Acetaminophen (N-acetyl-p-aminophenol, APAP) overdosing is among the leading causes of ALI and ALF in the Northern Hemisphere. Brain dysfunction defined as hepatic encephalopathy is one of the main diagnostic criteria for ALF. While neuroinflammation and brain metabolic alterations significantly contribute to hepatic encephalopathy, their evaluation at early stages of ALI remained challenging. To provide insights, we utilized post-mortem analysis and non-invasive brain micro positron emission tomography (microPET) imaging of mice with APAP-induced ALI.
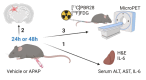
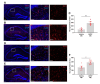
Methods: Male C57BL/6 mice were treated with vehicle or APAP (600 mg/kg, i.p.). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), liver damage (using H&E staining), hepatic and serum IL-6 levels, and hippocampal IBA1 (using immunolabeling) were evaluated at 24h and 48h. Vehicle and APAP treated animals also underwent microPET imaging utilizing a dual tracer approach, including [11C]-peripheral benzodiazepine receptor ([11C]PBR28) to assess microglia/astrocyte activation and [18F]-fluoro-2-deoxy-2-D-glucose ([18F]FDG) to assess energy metabolism. Brain images were pre-processed and evaluated using conjunction and individual tracer uptake analysis.
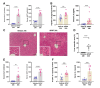
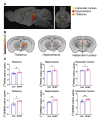
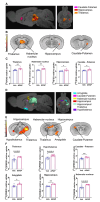
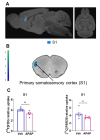
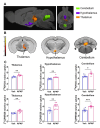
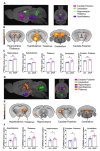
Results: APAP-induced ALI and hepatic and systemic inflammation were detected at 24h and 48h by significantly elevated serum ALT and AST levels, hepatocellular damage, and increased hepatic and serum IL-6 levels. In parallel, increased microglial numbers, indicative for neuroinflammation were observed in the hippocampus of APAP-treated mice. MicroPET imaging revealed overlapping increases in [11C]PBR28 and [18F]FDG uptake in the hippocampus, thalamus, and habenular nucleus indicating microglial/astroglial activation and increased energy metabolism in APAP-treated mice (vs. vehicle-treated mice) at 24h. Similar significant increases were also found in the hypothalamus, thalamus, and cerebellum at 48h. The individual tracer uptake analyses (APAP vs vehicle) at 24h and 48h confirmed increases in these brain areas and indicated additional tracer- and region-specific effects including hippocampal alterations.
Conclusion: Peripheral manifestations of APAP-induced ALI in mice are associated with brain neuroinflammatory and metabolic alterations at relatively early stages of disease progression, which can be non-invasively evaluated using microPET imaging and conjunction analysis. These findings support further PET-based investigations of brain function in ALI/ALF that may inform timely therapeutic interventions.
Keywords: acetaminophen; acute liver injury; brain; brain glucose metabolism; conjunction analysis; neuroinflammation; non-invasive microPET imaging.
Figures
References
-
- Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, et al. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver transplantation and surgery : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 1999;5(1):29–34. - PubMed
-
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Annals of internal medicine. 2002;137(12):947–54. - PubMed
-
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10(8):1018–23. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
