This is a preprint.
Interhemispheric CA1 projections support spatial cognition and are affected in a mouse model of the 22q11.2 deletion syndrome
- PMID: 39282348
- PMCID: PMC11398471
- DOI: 10.1101/2024.09.05.611389
Interhemispheric CA1 projections support spatial cognition and are affected in a mouse model of the 22q11.2 deletion syndrome
Abstract
Untangling the hippocampus connectivity is critical for understanding the mechanisms supporting learning and memory. However, the function of interhemispheric connections between hippocampal formations is still poorly understood. So far, two major hippocampal commissural projections have been characterized in rodents. Mossy cells from the hilus of the dentate gyrus project to the inner molecular layer of the contralateral dentate gyrus and CA3 and CA2 pyramidal neuron axonal collaterals to contralateral CA3, CA2 and CA1. In contrary, little is known about commissural projection from the CA1 region. Here, we show that CA1 pyramidal neurons from the dorsal hippocampus project to contralateral dorsal CA1 as well as dorsal subiculum. We further demonstrate that the interhemispheric projection from CA1 to dorsal subiculum supports spatial memory and spatial working memory in WT mice, two cognitive functions impaired in male mice from the Df16(A) +/- model of 22q11.2 deletion syndrome (22q11.2DS) associated with schizophrenia. Investigation of the CA1 interhemispheric projections in Df16(A) +/- mice revealed that these projections are disrupted with male mutants showing stronger anatomical defects compared to females. Overall, our results characterize a novel interhemispheric projection from dCA1 to dorsal subiculum and suggest that dysregulation of this projection may contribute to the cognitive deficits associated with the 22q11.2DS.
Figures
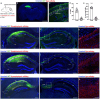


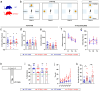


References
-
- Seoane L.F. (2023). Optimality Pressures toward Lateralization of Complex Brain Functions. Phys Rev X 13, 031028. 10.1103/PHYSREVX.13.031028/FIGURES/11/MEDIUM. - DOI
-
- Butler A.B., and Hodos W. (2005). Comparative Vertebrate Neuroanatomy: Evolution and Adaptation: Second Edition. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation: Second Edition, 1–715. 10.1002/0471733849. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
