This is a preprint.
Brain-wide circuitry underlying altered auditory habituation in zebrafish models of autism
- PMID: 39282371
- PMCID: PMC11398315
- DOI: 10.1101/2024.09.04.611137
Brain-wide circuitry underlying altered auditory habituation in zebrafish models of autism
Abstract
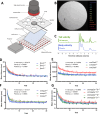
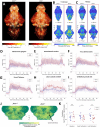
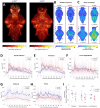
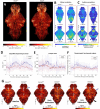
Auditory processing is widely understood to occur differently in autism, though the patterns of brain activity underlying these differences are not well understood. The diversity of autism also means brain-wide networks may change in various ways to produce similar behavioral outputs. We used larval zebrafish to investigate auditory habituation in four genetic lines relevant to autism: fmr1, mecp2, scn1lab and cntnap2. In free-swimming behavioral tests, we found each line had a unique profile of auditory hypersensitivity and/or delayed habituation. Combining the optical transparency of larval zebrafish with genetically encoded calcium indicators and light-sheet microscopy, we then observed brain-wide activity at cellular resolution during auditory habituation. As with behavior, each line showed unique alterations in brain-wide spontaneous activity, auditory processing, and adaptation in response to repetitive acoustic stimuli. We also observed commonalities in activity across our genetic lines that indicate shared circuit changes underlying certain aspects of their behavioral phenotypes. These were predominantly in regions involved in sensory integration and sensorimotor gating rather than primary auditory areas. Overlapping phenotypes include differences in the activity and functional connectivity of the telencephalon, thalamus, dopaminergic regions, and the locus coeruleus, and excitatory/inhibitory imbalance in the cerebellum. Unique phenotypes include loss of activity in the habenula in scn1lab, increased activity in auditory regions in fmr1, and differences in network activity over time in mecp2 and cntnap2. Comparing these distinct but overlapping brain-wide auditory networks furthers our understanding of how diverse genetic factors can produce similar behavioral effects through a range of circuit- and network-scale mechanisms.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
