This is a preprint.
AI-Based Discovery and CryoEM Structural Elucidation of a KATP Channel Pharmacochaperone
- PMID: 39282384
- PMCID: PMC11398524
- DOI: 10.1101/2024.09.05.611490
AI-Based Discovery and CryoEM Structural Elucidation of a KATP Channel Pharmacochaperone
Update in
-
AI-based discovery and cryoEM structural elucidation of a KATP channel pharmacochaperone.Elife. 2025 Mar 26;13:RP103159. doi: 10.7554/eLife.103159. Elife. 2025. PMID: 40135739 Free PMC article.
Abstract
Pancreatic KATP channel trafficking defects underlie congenital hyperinsulinism (CHI) cases unresponsive to the KATP channel opener diazoxide, the mainstay medical therapy for CHI. Current clinically used KATP channel inhibitors have been shown to act as pharmacochaperones and restore surface expression of trafficking mutants; however, their therapeutic utility for KATP trafficking impaired CHI is hindered by high-affinity binding, which limits functional recovery of rescued channels. Recent structural studies of KATP channels employing cryo-electron microscopy (cryoEM) have revealed a promiscuous pocket where several known KATP pharmacochaperones bind. The structural knowledge provides a framework for discovering KATP channel pharmacochaperones with desired reversible inhibitory effects to permit functional recovery of rescued channels. Using an AI-based virtual screening technology AtomNet® followed by functional validation, we identified a novel compound, termed Aekatperone, which exhibits chaperoning effects on KATP channel trafficking mutations. Aekatperone reversibly inhibits KATP channel activity with a half-maximal inhibitory concentration (IC50) ~ 9 μM. Mutant channels rescued to the cell surface by Aekatperone showed functional recovery upon washout of the compound. CryoEM structure of KATP bound to Aekatperone revealed distinct binding features compared to known high affinity inhibitor pharmacochaperones. Our findings unveil a KATP pharmacochaperone enabling functional recovery of rescued channels as a promising therapeutic for CHI caused by KATP trafficking defects.
Keywords: ABC transporter; ATP-sensitive potassium channel; Kir6.2; SUR1; cryoEM structure; drug discovery; pharmacological chaperones.
Figures


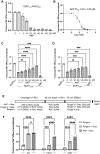
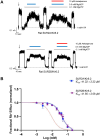


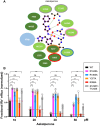
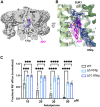
References
-
- Aguilar-Bryan L., & Bryan J. (1999). Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocrine reviews, 20(2), 101–135. - PubMed
-
- Ambrósio A. F., Silva A. P., Malva J. O., Soares-da-Silva P., Carvalho A. P., & Carvalho C. M. (1999). Carbamazepine inhibits L-type Ca2+ channels in cultured rat hippocampal neurons stimulated with glutamate receptor agonists. Neuropharmacology, 38(9), 1349–1359. 10.1016/s0028-3908(99)00058-1 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
