This is a preprint.
A flexible photoacoustic retinal prosthesis
- PMID: 39282448
- PMCID: PMC11398364
- DOI: 10.1101/2024.09.03.611068
A flexible photoacoustic retinal prosthesis
Update in
-
A flexible photoacoustic retinal prosthesis.Nat Commun. 2025 Dec 23;17(1):815. doi: 10.1038/s41467-025-67518-6. Nat Commun. 2025. PMID: 41436475 Free PMC article.
Abstract
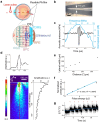
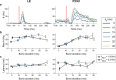
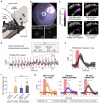
Retinal degenerative diseases of photoreceptors are a leading cause of blindness with no effective treatment. Retinal prostheses aim to restore sight by stimulating residual retinal cells. Here, we present a photoacoustic retinal stimulation technology. We designed a polydimethylsiloxane and carbon-based flexible film that converts near-infrared laser pulses into a localized acoustic field with 56-μm lateral resolution, allowing precise stimulation of mechanosensitive retinal cells. This photoacoustic stimulation robustly and locally modulated retinal ganglion cell activity in both wild-type and degenerated ex vivo rat retinae. In animals subretinally implanted with a millimeter-sized photoacoustic film , pulsed laser stimulation generated neural modulation along the visual pathway to the superior colliculus, as measured by functional ultrasound imaging. The biosafety of the film was confirmed by the absence of short-term adverse effects , while local thermal increases were measured below 1 °C. These findings demonstrate the potential of photoacoustic stimulation for high-acuity visual restoration in blind patients.
Conflict of interest statement
Competing interests This study was funded in part by the company Axorus SAS. J-DL and HM are major stakeholders in Axorus. CY and J-XC are minor stakeholders in Axorus.
Figures




References
-
- Li Jeany Q et al. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br. J. Ophthalmol. 104, 1077 (2020). - PubMed
-
- Muqit M. M. K. et al. Prosthetic Visual Acuity with the PRIMA Subretinal Microchip in Patients with Atrophic Age-Related Macular Degeneration at 4 Years Follow-up. Ophthalmol. Sci. 4, (2024).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
