Hypoxia-induced epigenetic regulation of breast cancer progression and the tumour microenvironment
- PMID: 39282472
- PMCID: PMC11392762
- DOI: 10.3389/fcell.2024.1421629
Hypoxia-induced epigenetic regulation of breast cancer progression and the tumour microenvironment
Abstract
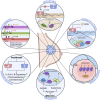
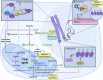
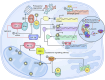
The events that control breast cancer progression and metastasis are complex and intertwined. Hypoxia plays a key role both in oncogenic transformation and in fueling the metastatic potential of breast cancer cells. Here we review the impact of hypoxia on epigenetic regulation of breast cancer, by interfering with multiple aspects of the tumour microenvironment. The co-dependent relationship between oxygen depletion and metabolic shift to aerobic glycolysis impacts on a range of enzymes and metabolites available in the cell, promoting posttranslational modifications of histones and chromatin, and changing the gene expression landscape to facilitate tumour development. Hormone signalling, particularly through ERα, is also tightly regulated by hypoxic exposure, with HIF-1α expression being a prognostic marker for therapeutic resistance in ER+ breast cancers. This highlights the strong need to understand the hypoxia-endocrine signalling axis and exploit it as a therapeutic target. Furthermore, hypoxia has been shown to enhance metastasis in TNBC cells, as well as promoting resistance to taxanes, radiotherapy and even immunotherapy through microRNA regulation and changes in histone packaging. Finally, several other mediators of the hypoxic response are discussed. We highlight a link between ionic dysregulation and hypoxia signalling, indicating a potential connection between HIF-1α and tumoural Na+ accumulation which would be worth further exploration; we present the role of Ca2+ in mediating hypoxic adaptation via chromatin remodelling, transcription factor recruitment and changes in signalling pathways; and we briefly summarise some of the findings regarding vesicle secretion and paracrine induced epigenetic reprogramming upon hypoxic exposure in breast cancer. By summarising these observations, this article highlights the heterogeneity of breast cancers, presenting a series of pathways with potential for therapeutic applications.
Keywords: breast cancer; epigenetics; hypoxia; microenvironment; oestrogen receptor; triple negative breast cancer.
Copyright © 2024 Capatina, Malcolm, Stenning, Moore, Bridge, Brackenbury and Holding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

