2 H-Thiazolo[4,5- d][1,2,3]triazole: synthesis, functionalization, and application in scaffold-hopping
- PMID: 39282646
- PMCID: PMC11391401
- DOI: 10.1039/d4sc03874f
2 H-Thiazolo[4,5- d][1,2,3]triazole: synthesis, functionalization, and application in scaffold-hopping
Abstract
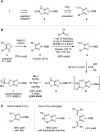
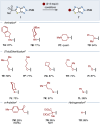
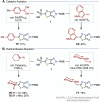
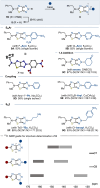
This manuscript unveils the synthesis of 2H-thiazolo[4,5-d][1,2,3]triazole (ThTz), an unprecedented [5-5]-fused heteroaromatic system, and established a scalable synthetic procedure for producing large quantities of the ThTz ring bearing a sulfone group on the thiazole ring. The sulfone moiety proves to be a versatile reactive tag, facilitating diverse transformations such as SNAr reactions, metal-catalyzed couplings, and radical-based alkylations. Furthermore, functionalization of the triazole ring highlights the potential of this newly developed heteroaromatic compound as a valuable heteroaryl building block, promoting scaffold hopping strategies in medicinal chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Whittamore P. R. O. Addie M. S. Bennett S. N. L. Birch A. M. Butters M. Godfrey L. Kenny P. W. Morley A. D. Murray P. M. Oikonomakos N. G. Otterbein L. R. Pannifer A. D. Parker J. S. Readman K. Siedlecki P. S. Schofield P. Stocker A. Taylor M. J. Townsend L. A. Whalley D. P. Whitehouse J. Bioorg. Med. Chem. Lett. 2006;16:5567–5571. doi: 10.1016/j.bmcl.2006.08.047. - DOI - PubMed
- Freeman S. Bartlett J. B. Convey G. Hardern I. Teague J. L. Loxham S. J. G. Allen J. M. Poucher S. M. Charles A. D. Br. J. Pharmacol. 2006;149:775–785. doi: 10.1038/sj.bjp.0706925. - DOI - PMC - PubMed
-
- Bhattacharya S. K. Andrews K. Beveridge R. Cameron K. O. Chen C. Dunn M. Fernando D. Gao H. Hepworth D. Jackson V. M. Khot V. Kong J. Kosa R. E. Lapham K. Loria P. M. Londregan A. T. McClure K. F. Orr S. T. M. Patel J. Rose C. Saenz J. Stock I. A. Storer G. VanVolkenburg M. Vrieze D. Wang G. Xiao J. Zhang Y. ACS Med. Chem. Lett. 2014;5:474–479. doi: 10.1021/ml400473x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

