Omalizumab reduces anaphylactic reactions and allows food introduction in food-allergic in children with severe asthma: An observational study
- PMID: 39282750
- PMCID: PMC11969307
- DOI: 10.1111/all.16314
Omalizumab reduces anaphylactic reactions and allows food introduction in food-allergic in children with severe asthma: An observational study
Abstract
Background: In Europe, Omalizumab (anti-IgE) is indicated for the treatment of moderate to severe asthma, but not for IgE-mediated food allergy (FA).
Objective: We assessed the impact of Omalizumab on efficacy, safety, and quality of life (FA-QoL) in patients with moderate to severe asthma and who have a history of anaphylaxis to peanut, tree nuts, fish, egg, milk, and/or wheat.
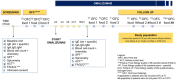
Methods: Food-allergic children (6-18 years) with moderate to severe asthma underwent oral food challenges (OFCs) to establish the threshold of reaction to the culprit food(s) at baseline (T0) and at 4-month intervals (T1, T2, and T3) during their first year of treatment with Omalizumab. We recorded the number and severity of food-allergic reactions, Asthma Control Test (ACT) scores, FA-QoL, and total IgE levels.
Results: In 65 patients allergic to 107 foods, the No Observed Adverse Events Level (NOAEL) at T1 increased: 243- and 488-fold for fresh and baked milk, respectively; 172- and 134-fold for raw and baked egg; 245-fold for hazelnut; 55-fold for peanut; 31-fold for wheat; and 10-fold for fish. Full tolerance was achieved in 66.4% of OFCs at T1, 58.3% at T2, and 75% at T3. Ninety-five foods were liberalized in the diet of 55 patients; the remaining 12 were introduced by 10 patients at least in traces. Throughout the study, 40 out of 65 were able to get a free diet. ACT increased from 17 (Q1-Q3: 15-17) to 23.6 (Q1-Q3: 23-25). The FA-QoL score in children ≤12 years decreased from 4.63 ± 0.74 to 2.02 ± 1.13, and in adolescents from 4.68 ± 0.92 to 1.90 ± 1.50.
Conclusions: During Omalizumab therapy, a safe reintroduction of allergenic foods is feasible.
Trial registration number: ClinicalTrials.gov, NCT06316414.
Keywords: IgE; allergy; anti IgE; asthma; children; food; immunologic desensitization; omalizumab.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
S.A. has participated as an advisory board member, and/or consultant, and/or speaker for Aimmune, D.B.V.‐, Novartis, Ferrero and Ulrich outside the submitted work. AF has participated as an advisory board member, and/or consultant, and/or speaker for Danone, Abbott, Aimmune, Ferrero, Novartis, outside the submitted work. Other Authors declare no conflict of interest related to this work.
Figures





References
-
- Sampath V, Abrams EM, Adlou B, et al. Food allergy across the globe. J Allergy Clin Immunol. 2021;148:1347‐1364. - PubMed
-
- Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141:41‐58. - PubMed
-
- Golding MA, Batac ALR, Gunnarsson NV, Ahlstedt S, Middelveld R, Protudjer JLP. The burden of food allergy on children and teens: a systematic review. Pediatr Allergy Immunol. 2022;33:e13743. - PubMed

