A Super-Ionic Solid-State Block Copolymer Electrolyte
- PMID: 39282822
- PMCID: PMC11618730
- DOI: 10.1002/smll.202404297
A Super-Ionic Solid-State Block Copolymer Electrolyte
Abstract
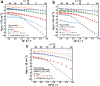
Polymer solid-state electrolytes offer great promise for battery materials with high energy density, mechanical stability, and improved safety. However, their low ion conductivities have so far limited their potential applications. Here, it is shown for poly(ethylene oxide) block copolymers that the super-stoichiometric addition of lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) as lithium salt leads to the formation of a crystalline PEO block copolymer phase with exceptionally high ion conductivities and low activation energies. The addition of LiTFSI further induces block copolymer phase transitions into bi-continuous Fddd and gyroid network morphologies, providing continuous 3D conduction pathways. Both effects lead to solid-state block copolymer electrolyte membranes with ion conductivities of up to 1·10-1 S cm-1 at 90 °C, decreasing only moderately to 4·10-2 S cm-1 at room temperature, and to >1·10-3 S cm-1 at -20 °C, corresponding to activation energies as low as 0.19 eV. The co-crystallization of PEO and LiTFSI with ether and carbonate solvents is observed to play a key role to realize a super-ionic conduction mechanism. The discovery of PEO super-ionic conductivity at high lithium concentrations opens a new pathway for fabrication of solid polymer electrolyte membranes with sufficiently high ion conductivities over a broad temperature range with widespread applications in electrical devices.
Keywords: block copolymer electrolytes; block copolymers; polyethylene oxide (PEO); solid‐state electrolytes; super‐ionic conductors.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Xu K., Chem. Rev. 2004, 104, 4303. - PubMed
-
- Kamaya N., Homma K., Yamakawa Y., Hirayama M., Kanno R., Yonemura M., Kamiyama T., Kato Y., Hama S., Kawamoto K., Mitsui A., Nat. Mater. 2011, 10, 682. - PubMed
-
- Zhao Q., Stalin S., Zhao C.‐Z., Archer L. A., Nat. Rev. Mater. 2020, 5, 229.
-
- Yang H., Wu N., Energy Sci. Eng. 2022, 10, 1643.
-
- Xue Z., He D., Xie X., J. Mater. Chem. A 2015, 3, 19218.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

