Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma
- PMID: 39282897
- PMCID: PMC12080919
- DOI: 10.1056/NEJMoa2407417
Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma
Abstract
Background: Previous results from this trial showed longer overall survival after treatment with nivolumab plus ipilimumab or with nivolumab monotherapy than with ipilimumab monotherapy in patients with advanced melanoma. Given that patients with advanced melanoma are living longer than 7.5 years, longer-term data were needed to address new clinically relevant questions.
Methods: We randomly assigned patients with previously untreated advanced melanoma, in a 1:1:1 ratio, to one of the following regimens: nivolumab (1 mg per kilogram of body weight) plus ipilimumab (3 mg per kilogram) every 3 weeks for four doses, followed by nivolumab (3 mg per kilogram) every 2 weeks; nivolumab (3 mg per kilogram) every 2 weeks plus placebo; or ipilimumab (3 mg per kilogram) every 3 weeks for four doses plus placebo. Treatment was continued until the occurrence of disease progression, unacceptable toxic effects, or withdrawal of consent. Randomization was stratified according to BRAF mutation status, metastasis stage, and programmed death ligand 1 expression. Here, we report the final, 10-year results of this trial, including results for overall survival and melanoma-specific survival, as well as durability of response.
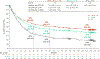
Results: With a minimum follow-up of 10 years, median overall survival was 71.9 months with nivolumab plus ipilimumab, 36.9 months with nivolumab, and 19.9 months with ipilimumab. The hazard ratio for death was 0.53 (95% confidence interval [CI], 0.44 to 0.65) for nivolumab plus ipilimumab as compared with ipilimumab and was 0.63 (95% CI, 0.52 to 0.76) for nivolumab as compared with ipilimumab. Median melanoma-specific survival was more than 120 months with nivolumab plus ipilimumab (not reached, with 37% of the patients alive at the end of the trial), 49.4 months with nivolumab, and 21.9 months with ipilimumab. Among patients who had been alive and progression-free at 3 years, 10-year melanoma-specific survival was 96% with nivolumab plus ipilimumab, 97% with nivolumab, and 88% with ipilimumab.
Conclusions: The final trial results showed a continued, ongoing survival benefit with nivolumab plus ipilimumab and with nivolumab monotherapy, as compared with ipilimumab monotherapy, in patients with advanced melanoma. (Funded by Bristol Myers Squibb and others; CheckMate 067 ClinicalTrials.gov number, NCT01844505.).
Copyright © 2024 Massachusetts Medical Society.
Figures









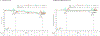
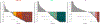
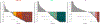
Comment in
-
Nivolumab plus Ipilimumab in Advanced Melanoma.N Engl J Med. 2025 Mar 27;392(12):1245-1246. doi: 10.1056/NEJMc2501311. N Engl J Med. 2025. PMID: 40138567 No abstract available.
-
Nivolumab plus Ipilimumab in Advanced Melanoma. Reply.N Engl J Med. 2025 Mar 27;392(12):1246. doi: 10.1056/NEJMc2501311. N Engl J Med. 2025. PMID: 40138568 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
