Effective Alkyl-Alkyl Cross-Coupling with an Iron-Xantphos Catalyst: Mechanistic and Structural Insights
- PMID: 39282992
- PMCID: PMC11701360
- DOI: 10.1002/anie.202413566
Effective Alkyl-Alkyl Cross-Coupling with an Iron-Xantphos Catalyst: Mechanistic and Structural Insights
Abstract
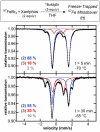
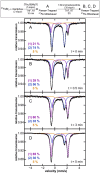
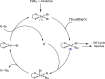
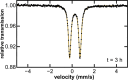
While iron-catalyzed C(sp2)-C(sp3) cross-couplings have been widely studied and developed in the last decade, alkyl-alkyl cross-coupling systems with iron remain underdeveloped despite the importance of C(sp3)-C(sp3) bonds in organic synthesis. A major challenge to the development of these reactions is the current lack of fundamental insight into ligand effects and organoiron intermediates that enable effective alkyl-alkyl couplings. The current study addresses this longstanding limitation using a combination of 57Fe Mössbauer spectroscopy, SC-XRD (single-crystal X-ray diffraction) and reactivity studies of alkyl-alkyl coupling with iron-Xantphos to define the in situ formed iron-Xantphos intermediates in catalysis. Combined with detailed reactivity studies, the nature of the key mechanistic pathways in catalysis and ligands effects to achieve effective alkyl-alkyl cross-coupling over competing β-H elimination pathways are probed. Overall, these foundational studies provide a platform for future bespoke ligand and pre-catalyst design for alkyl-alkyl cross-coupling methods development with sustainable iron catalysis.
Keywords: Cross-Coupling; Homogeneous Catalysis; Iron; Ligand Effects; Mechanism.
© 2024 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Kotha S., Lahiri K., Kashinath D., Tetrahedron 2002, 58, 9633–9695.
-
- Kadu B. S., Catalysis Science, Technology 2021, 11, 1186–1221.
-
- Tabassum S., Zahoor A. F., Ahmad S., Noreen R., Khan S. G., Ahmad H., Mol. Diversity 2022, 26, 647–689. - PubMed
-
- Nicolaou K. C., Bulger P. G., Sarlah D., Angew. Chem. Int. Ed. 2005, 44, 4442–4489. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

