Conjugation of anti-HIV gp41 monoclonal antibody to a drug capable of targeting resting lymphocytes produces an effective cytotoxic anti-HIV immunoconjugate
- PMID: 39283123
- PMCID: PMC11494876
- DOI: 10.1128/jvi.00647-24
Conjugation of anti-HIV gp41 monoclonal antibody to a drug capable of targeting resting lymphocytes produces an effective cytotoxic anti-HIV immunoconjugate
Abstract
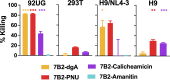
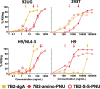
HIV-infected cells persisting in the face of suppressive antiretroviral therapy are the barrier to curing infection. Cytotoxic immunoconjugates targeted to HIV antigens on the cell surface may clear these cells. We showed efficacy in mouse and macaque models using immunotoxins, but immunogenicity blunted the effect. As an alternative, we propose antibody drug conjugates (ADCs), as used in cancer immunotherapy. In cancer, the target is a dividing cell, whereas it may not be in HIV. We screened cytotoxic drugs on human primary cells and cell lines. An anthracycline derivative, PNU-159682 (PNU), was highly cytotoxic to both proliferating and resting cells. Human anti-gp41 mAb 7B2 was conjugated to ricin A chain or PNU. The conjugates were tested in vitro for cytotoxic efficacy and anti-viral effect, and in vivo for tolerability. The specificity of killing for both conjugates was demonstrated on Env+ and Env- cells. The toxin conjugate was more potent and killed more rapidly, but 7B2-PNU was effective at levels achievable in patients. The ricin conjugate was well tolerated in mice; 7B2-PNU was toxic when administered intraperitoneally but was tolerated intravenously. We have produced an ADC with potential to target the persistent HIV reservoir in both dividing and non-dividing cells while avoiding immunogenicity. Cytotoxic anti-HIV immunoconjugates may have greatest utility as part of an "activate and purge" regimen, involving viral activation in the reservoir. This is a unique comparison of an immunotoxin and ADC targeted by the same antibody and tested in the same systems.IMPORTANCEHIV infection can be controlled with anti-retroviral therapy, but it cannot be cured. Despite years of therapy that suppresses HIV, patients again become viremic shortly after discontinuing treatment. A long-lived population of memory T cells retain the genes encoding HIV, and these cells secrete infectious HIV when no longer suppressed by therapy. This is the persistent reservoir of HIV infection. The therapies described here use anti-HIV antibodies conjugated to poisons to kill the cells in this reservoir. These poisons may be of several types, including protein toxins (immunotoxins) or anti-cancer drugs (antibody drug conjugates, ADCs). We have previously shown that an anti-HIV immunotoxin had therapeutic effects in animal models, but it elicited an anti-drug immune response. Here, we have prepared an anti-HIV ADC, which would be less likely to provoke an immune response, and show its potential for use in eliminating the persistent reservoir of HIV infection.
Keywords: HIV persistent reservoir; antibody drug conjugate; immunoconjugate; immunotoxin.
Conflict of interest statement
E.W.K., T.Z., and A.K. were employees of Lavena Biopharma at the time the experiments were performed.
Figures




References
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi:10.1038/8394 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

