Pharmacodynamic Response to Deucravacitinib, an Oral, Selective, Allosteric TYK2 Inhibitor, in a Global, Phase 2, Randomized, Double-Blind, Placebo-Controlled Psoriasis Trial
- PMID: 39283417
- PMCID: PMC11480296
- DOI: 10.1007/s13555-024-01262-5
Pharmacodynamic Response to Deucravacitinib, an Oral, Selective, Allosteric TYK2 Inhibitor, in a Global, Phase 2, Randomized, Double-Blind, Placebo-Controlled Psoriasis Trial
Abstract
Background: Psoriasis, a chronic, immune-mediated, inflammatory disease, affects 2‒3% of the population. Tyrosine kinase 2 (TYK2) mediates cytokine signaling involved in adaptive [interleukin (IL)-12, IL-23] and innate (type-I interferons) immune responses; IL-23-driven T-helper (Th)17 pathways play a key role in chronic inflammation in psoriasis. In a phase 2 trial, deucravacitinib, an oral, selective, allosteric TYK2 inhibitor, reduced IL-23/Th17 and type-I interferon pathway expression in the skin of patients with moderate to severe plaque psoriasis, reductions that were accompanied by clinical improvement of psoriatic lesions.
Objectives: The aim of this study was to identify biomarkers of psoriatic disease in serum from patients enrolled in the phase 2 trial and to assess the effects of deucravacitinib on those biomarkers.
Methods: Serum biomarkers from Olink proteomics and other quantitative assays were evaluated for a pharmacodynamic response to deucravacitinib treatment and correlation with psoriasis disease activity measures.
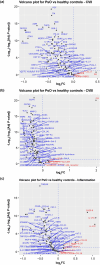
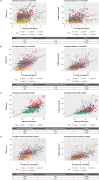
Results: Serum biomarkers associated with the IL-23/Th17 pathway [IL-17A, IL-17C, IL-19, IL-20, beta-defensin, and peptidase inhibitor 3 (PI3)] were upregulated in patients with psoriasis versus healthy controls. Deucravacitinib treatment reduced IL-17A (adjusted mean change from baseline at Day 85; 12 mg once daily versus placebo; -0.240 versus -0.067), IL-17C (-14.850 versus -1.664), IL-19 (-96.445 versus -8.119), IL-20 (-0.265 versus -0.064), beta-defensin (-65,025.443 versus -7553.961), and PI3 (-14.005 versus -1.360) expression. Reductions in serum biomarker expression occurred in a dose- and time-dependent manner, with significant reductions from baseline seen with deucravacitinib doses ≥ 3 mg twice daily (P ≤ 0.05). Biomarker expression correlated with disease activity measures such as Psoriasis Area and Severity Index (PASI) at baseline. Biomarker expression also correlated with PASI scores at Week 12.
Conclusion: IL-23/Th17 pathway expression in the serum of patients with psoriasis is an indicator of disease activity and response to deucravacitinib treatment.
Trial registration number: NCT02931838.
Keywords: Cytokine; Deucravacitinib; Pharmacodynamics; Psoriasis; Tyrosine kinase 2.
Plain language summary
Plaque psoriasis is a long-term disease that causes inflammation, scaling, and itching of the skin. Compared with healthy volunteers without psoriasis, patients with psoriasis have higher amounts of certain biomarkers (molecules that indicate what is happening in the body) in their blood that are associated with inflammation. Higher amounts of these biomarkers are also associated with more severe psoriasis. In a study of patients with psoriasis, those who received the oral drug deucravacitinib had lower amounts of biomarkers after 12 weeks of treatment compared with patients who received a placebo (a lookalike pill that contains no medicine). Patients who were treated with deucravacitinib also saw an improvement in their psoriasis after 12 weeks compared with patients who received placebo.
© 2024. The Author(s).
Conflict of interest statement
Ian M. Catlett was an employee of and shareholder in Bristol Myers Squibb at the time of study conduct. Lu Gao and Yanhua Hu are employees of and shareholders in Bristol Myers Squibb. Subhashis Banerjee was an employee of and shareholder in Bristol Myers Squibb at the time of study conduct. James G. Krueger has received research grants from Bristol Myers Squibb during the conduct of the study, personal fees from AbbVie, Baxter, Biogen, Delenex, Kineta, Sanofi, Serono, and Xenoport, and research grants from Amgen, Dermira, Innovaderm, Janssen, Kadmon, Kyowa, Lilly, Merck, Novartis, Paraxel, and Pfizer outside the submitted work.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
Medical

