iPSC-Derived LCHADD Retinal Pigment Epithelial Cells Are Susceptible to Lipid Peroxidation and Rescued by Transfection of a Wildtype AAV-HADHA Vector
- PMID: 39283617
- PMCID: PMC11407479
- DOI: 10.1167/iovs.65.11.22
iPSC-Derived LCHADD Retinal Pigment Epithelial Cells Are Susceptible to Lipid Peroxidation and Rescued by Transfection of a Wildtype AAV-HADHA Vector
Abstract
Purpose: Progressive choroid and retinal pigment epithelial (RPE) degeneration causing vision loss is a unique characteristic of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHADD), a fatty acid oxidation disorder caused by a common c.1528G>C pathogenic variant in HADHA, the α subunit of the mitochondrial trifunctional protein (TFP). We established and characterized an induced pluripotent stem cell (iPSC)-derived RPE cell model from cultured skin fibroblasts of patients with LCHADD and tested whether addition of wildtype (WT) HAHDA could rescue the phenotypes identified in LCHADD-RPE.
Methods: We constructed an rAAV expression vector containing 3' 3xFLAG-tagged human HADHA cDNA under the transcriptional control of the cytomegalovirus (CMV) enhancer-chicken beta actin (CAG) promoter (CAG-HADHA-3XFLAG). LCHADD-RPE were cultured, matured, and transduced with either AAV-GFP (control) or AAV-HADHA-3XFLAG.
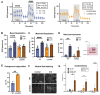
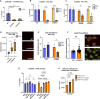
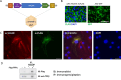
Results: LCHADD-RPE express TFP subunits and accumulate 3-hydroxy-acylcarnitines, cannot oxidize palmitate, and release fewer ketones than WT-RPE. When LCHADD-RPE are exposed to docosahexaenoic acid (DHA), they have increased oxidative stress, lipid peroxidation, decreased viability, and are rescued by antioxidant agents potentially explaining the pathologic mechanism of RPE loss in LCHADD. Transduced LCHADD-RPE expressing a WT copy of TFPα incorporated TFPα-FLAG into the TFP complex in the mitochondria and accumulated significantly less 3-hydroxy-acylcarnitines, released more ketones in response to palmitate, and were more resistant to oxidative stress following DHA exposure than control.
Conclusions: iPSC-derived LCHADD-RPE are susceptible to lipid peroxidation mediated cell death and are rescued by exogenous HADHA delivered with rAAV. These results are promising for AAV-HADHA gene addition therapy as a possible treatment for chorioretinopathy in patients with LCHADD.
Conflict of interest statement
Disclosure:
Figures


References
-
- Vockley J, Bennett MJ, Gillingham MB.. Mitochondrial fatty acid oxidation disorders. In: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA (eds), The Online Metabolic and Molecular Bases of Inherited Disease . New York, NY: McGraw-Hill Education; 2019.
-
- Ijlst L, Ruiter JP, Hoovers JM, Jakobs ME, Wanders RJ.. Common missense mutation G1528C in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Characterization and expression of the mutant protein, mutation analysis on genomic DNA and chromosomal localization of the mitochondrial trifunctional protein alpha subunit gene. J Clin Invest. 1996; 98: 1028–1033. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

