Mycophenolate Mofetil and New-Onset Systemic Lupus Erythematosus: A Randomized Clinical Trial
- PMID: 39283640
- PMCID: PMC11406395
- DOI: 10.1001/jamanetworkopen.2024.32131
Mycophenolate Mofetil and New-Onset Systemic Lupus Erythematosus: A Randomized Clinical Trial
Abstract
Importance: Anti-double-stranded DNA (dsDNA) antibody has been reported to have a close relationship with systemic lupus erythematosus (SLE) flares and participates in the pathogenesis of lupus nephritis (LN) as well as causing damage to other organs. However, whether early use of mycophenolate mofetil (MMF) could prevent SLE flares is not clear.
Objective: To assess the efficacy and safety of MMF plus prednisone and hydroxychloroquine sulfate compared with prednisone and hydroxychloroquine sulfate alone in patients with SLE.
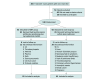
Design, setting, and participants: This investigator-initiated, multicenter, observer-blinded randomized clinical trial enrolled 130 participants aged 18 to 65 years and was conducted in 3 hospitals across China. Treatment-naive patients with newly diagnosed SLE, a high titer of anti-dsDNA antibody, and no major organ involvement were included. The study was started September 1, 2018, and the follow-up was completed September 30, 2021. Data were analyzed from December 1, 2021, to March 31, 2022.
Interventions: Patients were randomized 1:1 to receive oral prednisone (0.5 mg/kg/d) and hydroxychloroquine sulfate (5 mg/kg/d) (control group) or prednisone (0.5 mg/kg/d) and hydroxychloroquine sulfate (5 mg/kg/d) plus MMF (500 mg twice daily) (MMF group) for 96 weeks.
Main outcomes and measures: The primary outcome was the proportion of patients presenting with flares according to the Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) Flare Index. The secondary outcomes included the proportion with lupus low disease activity state at week 96, 36-Item Short Form Health Survey scores before and after treatment, proportion of adverse events (AEs), and changes in SLEDAI-2000 scores and prednisone doses.
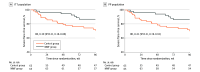
Results: Among 130 randomized patients (mean [SD] age, 34.5 [12.5] years; 112 [86.2%] women), 119 (91.5%) completed the follow-up. The risk of severe flare was significantly lower in the MMF group (7 of 65 [10.8%]) vs the control group (18 of 65 [27.7%]) (relative risk [RR], 0.39 [95% CI, 0.17-0.87]; P = .01). Additionally, 1 of 65 patients in the MMF group (1.5%) and 9 of 65 in the control group (13.8%) manifested LN (RR, 0.11 [95% CI, 0.01-0.85]; P = .008). Most common serious study drug-related AEs were infections (20 of 65 [30.8%] in the control group and 22 of 65 [33.8%] in the MMF group).
Conclusions and relevance: The findings of this randomized clinical trial suggest that MMF may reduce the rate of severe flare and lower the incidence of LN in patients with new-onset SLE and a high titer of anti-dsDNA antibody without major organ involvement.
Trial registration: Chinese Clinical Trial Registry: ChiCTR1800017540.
Conflict of interest statement
Figures
References
-
- Andrejevic S, Jeremic I, Sefik-Bukilica M, Nikolic M, Stojimirovic B, Bonaci-Nikolic B. Immunoserological parameters in SLE: high-avidity anti-dsDNA detected by ELISA are the most closely associated with the disease activity. Clin Rheumatol. 2013;32(11):1619-1626. doi: 10.1007/s10067-013-2330-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

