Effect of High-Intensity vs Low-Intensity Noninvasive Positive Pressure Ventilation on the Need for Endotracheal Intubation in Patients With an Acute Exacerbation of Chronic Obstructive Pulmonary Disease: The HAPPEN Randomized Clinical Trial
- PMID: 39283649
- PMCID: PMC11406453
- DOI: 10.1001/jama.2024.15815
Effect of High-Intensity vs Low-Intensity Noninvasive Positive Pressure Ventilation on the Need for Endotracheal Intubation in Patients With an Acute Exacerbation of Chronic Obstructive Pulmonary Disease: The HAPPEN Randomized Clinical Trial
Abstract
Importance: The effect of high-intensity noninvasive positive pressure ventilation (NPPV) on the need for endotracheal intubation in patients with an acute exacerbation of chronic obstructive pulmonary disease (COPD) is unknown.
Objective: To determine whether the use of high-intensity NPPV vs low-intensity NPPV reduces the need for endotracheal intubation in patients with an acute exacerbation of COPD and hypercapnia.
Design, setting, and participants: Randomized clinical trial conducted at 30 general respiratory non-intensive care unit wards of Chinese hospitals from January 3, 2019, to January 31, 2022; the last 90-day follow-up was on April 22, 2022. The included patients had an acute exacerbation of COPD and a Paco2 level greater than 45 mm Hg after receiving 6 hours of low-intensity NPPV.
Interventions: Patients were randomized 1:1 to receive high-intensity NPPV with inspiratory positive airway pressure that was adjusted to obtain a tidal volume 10 mL/kg to 15 mL/kg of predicted body weight (n = 147) or to continue receiving low-intensity NPPV with inspiratory positive airway pressure that was adjusted to obtain a tidal volume of 6 mL/kg to 10 mL/kg of predicted body weight (n = 153). Patients in the low-intensity NPPV group who met the prespecified criteria for the need for endotracheal intubation were allowed to crossover to high-intensity NPPV.
Main outcomes and measures: The primary outcome was the need for endotracheal intubation during hospitalization, which was defined by prespecified criteria. There were 15 prespecified secondary outcomes, including endotracheal intubation.
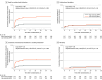
Results: The trial was terminated by the data and safety monitoring board and the trial steering committee after an interim analysis of the first 300 patients. Among the 300 patients who completed the trial (mean age, 73 years [SD, 10 years]; 68% were men), all were included in the analysis. The primary outcome of meeting prespecified criteria for the need for endotracheal intubation occurred in 7 of 147 patients (4.8%) in the high-intensity NPPV group vs 21 of 153 (13.7%) in the low-intensity NPPV group (absolute difference, -9.0% [95% CI, -15.4% to -2.5%], 1-sided P = .004). However, rates of endotracheal intubation did not significantly differ between groups (3.4% [5/147] in the high-intensity NPPV group vs 3.9% [6/153] in the low-intensity NPPV group; absolute difference, -0.5% [95% CI, -4.8% to 3.7%], P = .81). Abdominal distension occurred more frequently in the high-intensity NPPV group (37.4% [55/147]) compared with the low-intensity NPPV group (25.5% [39/153]).
Conclusions and relevance: Patients with COPD and persistent hypercapnia in the high-intensity NPPV group (vs patients in the low-intensity NPPV group) were significantly less likely to meet criteria for the need for endotracheal intubation; however, patients in the low-intensity NPPV group were allowed to crossover to high-intensity NPPV, and the between-group rate of endotracheal intubation was not significantly different.
Trial registration: ClinicalTrials.gov Identifier: NCT02985918.
Conflict of interest statement
Figures


Comment in
References
-
- Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174(12):1982-1993. doi: 10.1001/jamainternmed.2014.5430 - DOI - PMC - PubMed
-
- Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7(7):CD004104. doi: 10.1002/14651858.CD004104.pub4 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

