Bidirectional Mendelian Randomization Highlights Causal Relationships Between Circulating INHBC and Multiple Cardiometabolic Diseases and Traits
- PMID: 39283655
- PMCID: PMC11579406
- DOI: 10.2337/db24-0168
Bidirectional Mendelian Randomization Highlights Causal Relationships Between Circulating INHBC and Multiple Cardiometabolic Diseases and Traits
Abstract
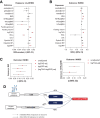
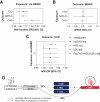
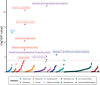
Human genetic and transgenic mouse studies have highlighted a potential liver-adipose tissue endocrine axis, involving activin C (Act-C) and/or Act-E and ALK7, influencing fat distribution and systemic metabolism. We investigated the bidirectional effects between circulating INHBC, which homodimerizes into Act-C, and adiposity traits, insulin resistance, inflammation, and cardiometabolic disease risk. Additionally, we examined whether Act-C is an ALK7 ligand in human adipocytes. We used Mendelian randomization and in vitro studies in immortalized human abdominal and gluteal adipocytes. Circulating INHBC was causally linked to reduced lower-body fat, dyslipidemia, and increased risks of coronary artery disease (CAD) and nonalcoholic fatty liver disease (NAFLD). Conversely, upper-body fat distribution, obesity, hypertriglyceridemia, subclinical inflammation, and type 2 diabetes positively impacted plasma INHBC levels. Mechanistically, an atherogenic lipid profile may partly explain the INHBC-CAD link, while inflammation and hypertriglyceridemia may partly explain how adiposity traits affect circulating INHBC. Phenome-wide Mendelian randomization showed weak causal relationships between higher plasma INHBC and impaired kidney function and higher gout risk. In human adipocytes, recombinant Act-C activated SMAD2/3 signaling via ALK7 and suppressed lipolysis. In summary, INHBC influences systemic metabolism by activating ALK7 in adipose tissue and may serve as a drug target for atherogenic dyslipidemia, CAD, and NAFLD.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Danforth E Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet 2000;26:13 - PubMed
-
- Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome–an allostatic perspective. Biochim Biophys Acta 2010;1801:338–349 - PubMed
-
- Abifadel M, Varret M, Rabès J-P, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003;34:154–156 - PubMed
-
- Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–1272 - PubMed
-
- Sabatine MS, Giugliano RP, Keech AC, et al.; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

