GDF-15 Predicts Epithelioid Hemangioendothelioma Aggressiveness and Is Downregulated by Sirolimus through ATF4/ATF5 Suppression
- PMID: 39283723
- PMCID: PMC11565171
- DOI: 10.1158/1078-0432.CCR-23-3991
GDF-15 Predicts Epithelioid Hemangioendothelioma Aggressiveness and Is Downregulated by Sirolimus through ATF4/ATF5 Suppression
Abstract
Purpose: Epithelioid hemangioendothelioma (EHE), an ultra-rare sarcoma, poses therapeutic challenges because of limited efficacy of conventional chemotherapy in advanced cases, necessitating exploration of new treatment avenues and identification of novel aggressive biomarkers. This study aimed at (i) utilizing a patient-derived xenograft model of EHE and its associated cell line to assess the efficacy of sirolimus and (ii) analyzing two distinct patient cohorts to pinpoint circulating biomarkers of EHE aggressiveness.
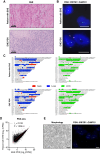
Experimental design: A patient-derived xenograft model and corresponding cell line were established from a patient with advanced EHE, demonstrating consistency with the original tumor in terms of histomorphology, WWTR1::CAMTA1 fusion presence, and genomic and transcriptomic profiles. Two independent patient series were employed to investigate the association between growth/differentiation factor 15 (GDF-15) serum levels and EHE aggressiveness.
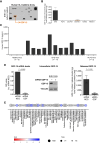
Results: ELISA analyses on EHE cell culture medium and blood from EHE-carrying mice revealed the release of GDF-15 by EHE cells. Sirolimus exhibited markedly higher antitumor activity compared with doxorubicin, concurrently reducing GDF-15 expression/release both in vivo and in vitro. This reduction was attributed to the drug-induced inhibition of phosphorylation/activation of 4E-BP1 and subsequent downregulation of the GDF-15 transcription factors ATF4 and ATF5. Blood sample analyses from two independent patient series showed a significant correlation between GDF-15 and EHE aggressiveness.
Conclusions: This study identifies GDF-15 as a novel biomarker of EHE aggressiveness and underscores the superior efficacy of sirolimus compared with doxorubicin in our experimental models. The observed inhibition of GDF-15 release by sirolimus suggests its potential as a biomarker for monitoring the drug's activity in patients.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S. Stacchiotti reports grants from Associazione Italiana per la Ricerca sul Cancro, International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as personal fees from Ikena and Astex Pharmaceuticals and personal fees and other support from Novartis outside the submitted work. S. Pasquali reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. A.M. Frezza reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as other support from Advenchen Laboratories, Amgen Dompé, AROG Pharmaceuticals, Ayala Pharmaceuticals, Bayer, Blueprint Medicines, Boehringer Ingelheim, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly and Company, Epizyme, Foghorn Therapeutics Inc., GlaxoSmithKline, Hutchison MediPharma Limited, Inhibrx, Karyopharm Therapeutics Inc., Novartis, Pfizer, PharmaMar, PTC Therapeutics, Rain Oncology, and SpringWorks Therapeutics outside the submitted work. A. Beretta reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. S. Percio reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. M. Lecchi reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. M. Tortoreto reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. A. Merlini reports other support from PharmaMar and personal fees from Boehringer Ingelheim outside the submitted work. P.H. Huang reports grants from EHE Rare Cancer Charity during the conduct of the study. W.D. Tap reports personal fees from Eli Lilly and Company, C4 Therapeutics, Daiichi Sankyo, Deciphera, Servier, Bayer, Cogent, Foghorn, Amgen, AmMax, Boehringer Ingelheim, BioAtla, Inhibrx, PharmaEssentia, Avacta, Ipsen, Sonata, Abbisko, Aadi, IMGT, and Ikena outside the submitted work as well as a patent for Companion Diagnostic for CDK4 inhibitors – 14/854329 pending to MSKCC/SKI and a patent for Enigma and CDH18 as Companion Diagnostics for CDK4 inhibition – SKI2016-021-03 issued to MSKCC/SKI. In addition, W.D. Tap reports membership on scientific advisory boards for Certis Oncology Solutions and Avacta, is a cofounder and shareholder of Atropos Therapeutics, is a shareholder and scientific advisory board member of Innova Therapeutics, and is a strategic advisory board member and chair of Osteosarcoma Institute. M. Ingrosso reports personal fees from Novartis outside the submitted work. C. Morosi reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID#24297)/Cancer Research UK (c56167/A29363)/Fundacion Cientifica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. C. Giani reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. P. Verderio reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. P.G. Casali reports grants from International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), Associazione Italiana per la Ricerca sul Cancro (IG 29477), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work; in addition, P.G. Casali is the chair of a unit that received research grants from Advenchen Laboratories, Amgen Dompé, AROG Pharmaceuticals, Bayer, Blueprint Medicines, Boehringer Ingelheim, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly and Company, Epizyme Inc., Foghorn Therapeutics Inc., GlaxoSmithKline, Hutchinson MediPharma Ltd., Inhibrx Inc., Karyopharm Therapeutics Inc., PTC Therapeutics, Novartis, Pfizer, PharmaMar, Rain Oncology, and SpringWorks Therapeutics. V. Zuco reports grants from Associazione Italiana per la Ricerca sul Cancro (IG 29477), International Accelerator Award funded by Associazione Italiana per la Ricerca sul Cancro (ID #24297)/Cancer Research UK (C56167/A29363)/Fundacion Científica, Asociación Española Contra el Cáncer (Foundation AECC-GEACC19007MA), EHE Rare Cancer Charity, and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. N. Zaffaroni reports grants from EHE Rare Cancer Charity and The EHE Foundation during the conduct of the study, as well as grants from Ikena Oncology and Astex Pharmaceuticals outside the submitted work. No disclosures were reported by the other authors.
Figures



References
-
- Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med 2011;3:98ra82. - PubMed

