The structural and functional effects of myosin regulatory light chain phosphorylation are amplified by increases in sarcomere length and [Ca2+]
- PMID: 39283968
- PMCID: PMC11466700
- DOI: 10.1113/JP286802
The structural and functional effects of myosin regulatory light chain phosphorylation are amplified by increases in sarcomere length and [Ca2+]
Abstract
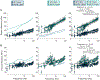
Precise regulation of sarcomeric contraction is essential for normal cardiac function. The heart must generate sufficient force to pump blood throughout the body, but either inadequate or excessive force can lead to dysregulation and disease. Myosin regulatory light chain (RLC) is a thick-filament protein that binds to the neck of the myosin heavy chain. Post-translational phosphorylation of RLC (RLC-P) by myosin light chain kinase is known to influence acto-myosin interactions, thereby increasing force production and Ca2+-sensitivity of contraction. Here, we investigated the role of RLC-P on cardiac structure and function as sarcomere length and [Ca2+] were altered. We found that at low, non-activating levels of Ca2+, RLC-P contributed to myosin head disorder, though there were no effects on isometric stress production and viscoelastic stiffness. With increases in sarcomere length and Ca2+-activation, the structural changes due to RLC-P become greater, which translates into greater force production, greater viscoelastic stiffness, slowed myosin detachment rates and altered nucleotide handling. Altogether, these data suggest that RLC-P may alter thick-filament structure by releasing ordered, off-state myosin. These more disordered myosin heads are available to bind actin, which could result in greater force production as Ca2+ levels increase. However, prolonged cross-bridge attachment duration due to slower ADP release could delay relaxation long enough to enable cross-bridge rebinding. Together, this work further elucidates the effects of RLC-P in regulating muscle function, thereby promoting a better understanding of thick-filament regulatory contributions to cardiac function in health and disease. KEY POINTS: Myosin regulatory light chain (RLC) is a thick-filament protein in the cardiac sarcomere that can be phosphorylated (RLC-P), and changes in RLC-P are associated with cardiac dysfunction and disease. This study assesses how RLC-P alters cardiac muscle structure and function at different sarcomere lengths and calcium concentrations. At low, non-activating levels of Ca2+, RLC-P contributed to myofilament disorder, though there were no effects on isometric stress production and viscoelastic stiffness. With increases in sarcomere length and Ca2+-activation, the structural changes due to RLC-P become greater, which translates into greater force production, greater viscoelastic stiffness, slower myosin detachment rate and altered cross-bridge nucleotide handling rates. This work elucidates the role of RLC-P in regulating muscle function and facilitates understanding of thick-filament regulatory protein contributions to cardiac function in health and disease.
Keywords: X‐ray diffraction; muscle mechanics; myofilament phosphorylation.
© 2024 The Authors. The Journal of Physiology © 2024 The Physiological Society.
Conflict of interest statement
Competing interests
T.I and W.M provides consulting and collaborative research studies to Edgewise Therapeutics, but such work is unrelated to the content of this article.
Figures







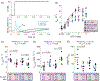
References
-
- Akiyama K, Akopian G, Jinadasa P, Gluckman TL, Terhakopian A, Massey B & Bing RJ (1997). Myocardial infarction and regulatory myosin light chain. J Mol Cell Cardiol 29, 2641–2652. - PubMed
-
- de Beer EL, Grundeman RL, Wilhelm AJ, van den Berg C, Caljouw CJ, Klepper D & Schiereck P (1988). Effect of sarcomere length and filament lattice spacing on force development in skinned cardiac and skeletal muscle preparations from the rabbit. Basic Res Cardiol 83, 410–423. - PubMed
MeSH terms
Substances
Grants and funding
- HL128368/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P30 AR074990/AR/NIAMS NIH HHS/United States
- R01HL171657/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL149164/HL/NHLBI NIH HHS/United States
- GM131981/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 HL128368/HL/NHLBI NIH HHS/United States
- 23TPA1074093/American Heart Association
- R01 HL171657/HL/NHLBI NIH HHS/United States
- P30 GM138395/GM/NIGMS NIH HHS/United States
- 2312925/National Science Foundation
- DE-AC02-06CH11357/U.S. Department of Energy
- RM1 GM131981/GM/NIGMS NIH HHS/United States
- R01HL149164/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Miscellaneous

