Real-world phenotyping and risk assessment of childhood asthma burden using national registries
- PMID: 39284553
- PMCID: PMC12079142
- DOI: 10.1016/j.rmed.2024.107808
Real-world phenotyping and risk assessment of childhood asthma burden using national registries
Abstract
Background: Phenotype classification contributes to risk assessment of asthma. Previous studies have applied this concept primarily to adult populations and in the setting of research protocol assessments which may not be applicable to clinical settings.
Objective: Exploring the value of routinely collected clinical data for phenotype classification and risk assessment of childhood asthma.
Methods: Using hospital and laboratory data, 29,851 children in a Danish nationwide database aged 2-17 years with ICS-treated asthma in 2015 followed for two years (730 days) were classified to have T2 (elevated blood eosinophils (>300 cells/μL) and/or elevated total- or specific-IgE), and/or non-T2 risk factors (in utero tobacco exposure and/or severe viral infections). Logistic regression was applied to quantify associations of risk factors with asthma severity, control, and exacerbation risk.
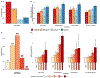
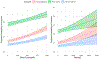
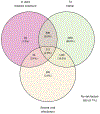
Results: In a complete case analysis, 85.8 % children had at least one T2 risk factor and 29.3 % had mixed T2/non-T2 risk factors. Elevated blood eosinophils and total/specific IgE were associated with exacerbations (ORs 1.55 (1.38-1.73) and 1.41 (1.20-1.66) and higher asthma severity (1.42 (1.24-1.63) and 1.31 (1.08-1.60)), respectively. Dose-dependency was observed between blood eosinophil counts, total IgE levels, and risk of adverse outcomes. Furthermore, accumulation of risk factors demonstrated an increasing risk, with children with all four risk factors having a high risk of any adverse asthma-related outcome (OR 3.13 (2.03-4.82) CONCLUSION: Asthma phenotypic markers defined in research protocols can be reliably applied in real-world settings by utilizing data collected during routine clinical care and enable better classification of risk of adverse asthma outcomes.
Keywords: Eosinophils; Exacerbations; Pharmacoepidemiology; Phenotypes; Severe asthma.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest KEJH has received personal fees from AstraZeneca, Chiesi, GSK, Sanofi and TEVA. NA, SCG declares no conflicts of interest. DR declares no conflicts of interest but does serve on a DSMB for the National Institute of Health. CSU has received personal fees from AstraZeneca, GSK, TEVA, Chiesi, Sanofi Genzyme, Boehringer-Ingelheim, Orion Pharma, Novartis, ALK-Abello, Mundipharma, Berlin Chemie, Pfizer, TFF Pharmaceuticals, Covis Pharma and Actelion. VB has received personal fees from AstraZeneca, GSK, TEVA, Sanofi Genzyme, MSD, Chiesi, Boehringer-Ingelheim, Novartis, ALK-Abello, Mundipharma, BIRK NPC and Pharmaxis.
Figures

References
-
- Pijnenburg MW, Frey U, De Jongste JC, Saglani S. Childhood asthma: pathogenesis and phenotypes. Eur Respir J. 2022. Jun;59(6). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

