Therapy-related AML: long-term outcome in a large cohort of AML-patients with intensive and non-intensive therapy
- PMID: 39284846
- PMCID: PMC11405931
- DOI: 10.1038/s41408-024-01140-5
Therapy-related AML: long-term outcome in a large cohort of AML-patients with intensive and non-intensive therapy
Abstract
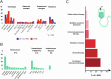
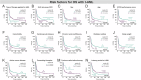
Therapy-related acute myeloid leukemia (t-AML) often exhibits adverse (genetic) features. There is ongoing discussion on the impact of t-AML on long-term outcome in AML. Therefore, we retrospectively analyzed clinical and biological characteristics of 1133 AML patients (225 t-AML patients and 908 de novo AML patients) with a median follow-up of 81.8 months. T-AML patients showed more adverse genetic alterations, higher age and more comorbidities as compared to de novo AML. Median OS in intensively treated t-AML patients was 13.7 months as compared to 39.4 months in de novo AML (p < 0.001). With non-intensive therapy, OS did not differ significantly (p = 0.394). With intensive therapy, significant differences in favor of de novo AML were observed in the ELN intermediate I/II (p = 0.009) and adverse (p = 0.016) risk groups but not within favorable risk groups (APL p = 0.927, ELN favorable p = 0.714). However, t-AML was no independent risk factor for OS (p = 0.103), RR (p = 0.982) and NRM (p = 0.320) in the multivariate analysis. A limitation of our study is an ELN 2010 risk stratification due to a lack of more comprehensive molecular data according to ELN 2022. We conclude that therapeutic algorithms in t-AML, in particular with regard to allo-HSCT, should be guided by ELN genetic risk rather than classification as t-AML alone. Our data support the WHO and ICC 2022 classifications, which include t-AML as diagnostic qualifier rather than a separate subcategory.
© 2024. The Author(s).
Conflict of interest statement
JI: travel support from Medac. AF: honoraria from Pharmaphar; Ipsen; Lilly. JK: honoraria from Janssen, BMS/Celgene, Takeda; Astra Zeneca, Amgen; Sanofi Aventis; Abbvie. PLC: honoraria from Pfizer; Incyte; Novartis. UK: Honoraria from Novartis. LB: honoraria from AbbVie, Amgen, Astellas, BristolMyers Squibb, Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer, Roche, and Sanofi, as well as research support from Bayer and Jazz Pharmaceuticals. JW: honoraria from Abbvie, Pfizer, Novartis; Jazz Pharmaceuticals, Roche; Astellas; Amgen; Sanofi Aventis; Bristol Myers Squibb, SoBi, Otsuka. The authors declare no competing interests.
Figures




References
-
- Andersen MK, Johansson B, Larsen SO, Pedersen-Bjergaard J. Chromosomal abnormalities in secondary MDS and AML. Relationship to drugs and radiation with specific emphasis on the balanced rearrangements. Haematologica. 1998;83:483–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

