N1-Methylpseudouridine and pseudouridine modifications modulate mRNA decoding during translation
- PMID: 39284850
- PMCID: PMC11405884
- DOI: 10.1038/s41467-024-51301-0
N1-Methylpseudouridine and pseudouridine modifications modulate mRNA decoding during translation
Abstract
The ribosome utilizes hydrogen bonding between mRNA codons and aminoacyl-tRNAs to ensure rapid and accurate protein production. Chemical modification of mRNA nucleobases can adjust the strength and pattern of this hydrogen bonding to alter protein synthesis. We investigate how the N1-methylpseudouridine (m1Ψ) modification, commonly incorporated into therapeutic and vaccine mRNA sequences, influences the speed and fidelity of translation. We find that m1Ψ does not substantially change the rate constants for amino acid addition by cognate tRNAs or termination by release factors. However, we also find that m1Ψ can subtly modulate the fidelity of amino acid incorporation in a codon-position and tRNA dependent manner in vitro and in human cells. Our computational modeling shows that altered energetics of mRNA:tRNA interactions largely account for the context dependence of the low levels of miscoding we observe on Ψ and m1Ψ containing codons. The outcome of translation on modified mRNA bases is thus governed by the sequence context in which they occur.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
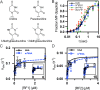

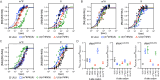

References
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008602/GM/NIGMS NIH HHS/United States
- Cottrell Scholar/Research Corporation for Science Advancement (Research Corporation)
- R01 GM093278/GM/NIGMS NIH HHS/United States
- CAREER 2045562/National Science Foundation (NSF)
- R01GM093278/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
LinkOut - more resources
Full Text Sources

