Potent combination benefit of the AKT inhibitor capivasertib and the BCL-2 inhibitor venetoclax in diffuse large B cell lymphoma
- PMID: 39284898
- PMCID: PMC11588655
- DOI: 10.1038/s41375-024-02401-9
Potent combination benefit of the AKT inhibitor capivasertib and the BCL-2 inhibitor venetoclax in diffuse large B cell lymphoma
Abstract
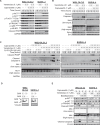
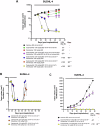
The therapeutic potential of targeting PI3K/AKT/PTEN signalling in B-cell malignancies remains attractive. Whilst PI3K-α/δ inhibitors demonstrate clinical benefit in certain B-cell lymphomas, PI3K signalling inhibitors have been inadequate in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) in part, due to treatment related toxicities. Clinically, AKT inhibitors exhibit a differentiated tolerability profile offering an alternative approach for treating patients with B-cell malignancies. To explore how AKT inhibition complements other potential therapeutics in the treatment of DLBCL patients, an in vitro combination screen was conducted across a panel of DLCBL cell lines. The AKT inhibitor, capivasertib, in combination with the BCL-2 inhibitor, venetoclax, produced notable therapeutic benefit in preclinical models of DLBCL. Capivasertib and venetoclax rapidly induced caspase and PARP cleavage in GCB-DLBCL PTEN wildtype cell lines and those harbouring PTEN mutations or reduced PTEN protein, driving prolonged tumour growth inhibition in DLBCL cell line and patient derived xenograft lymphoma models. The addition of the rituximab further deepened the durability of capivasertib and venetoclax responses in a RCHOP refractory DLBCL in vivo models. These findings provide preclinical evidence for the rational treatment combination of AKT and BCL-2 inhibitors using capivasertib and venetoclax respectively alongside anti-CD20 antibody supplementation for treatment of patients with DLBCL.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: B.W., K.M., H.D., I.N., A.R., M.P., N.B., J.R.R., C.R., J.M., and S.B. are current or former AstraZeneca employees and shareholders.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

