Enhanced complement activation and MAC formation accelerates severe COVID-19
- PMID: 39284944
- PMCID: PMC11405604
- DOI: 10.1007/s00018-024-05430-w
Enhanced complement activation and MAC formation accelerates severe COVID-19
Abstract
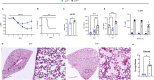
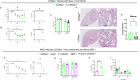
Emerging evidence indicates that activation of complement system leading to the formation of the membrane attack complex (MAC) plays a detrimental role in COVID-19. However, their pathogenic roles have never been experimentally investigated before. We used three knock out mice strains (1. C3-/-; 2. C7-/-; and 3. Cd59ab-/-) to evaluate the role of complement in severe COVID-19 pathogenesis. C3 deficient mice lack a key common component of all three complement activation pathways and are unable to generate C3 and C5 convertases. C7 deficient mice lack a complement protein needed for MAC formation. Cd59ab deficient mice lack an important inhibitor of MAC formation. We also used anti-C5 antibody to block and evaluate the therapeutic potential of inhibiting MAC formation. We demonstrate that inhibition of complement activation (in C3-/-) and MAC formation (in C3-/-. C7-/-, and anti-C5 antibody) attenuates severe COVID-19; whereas enhancement of MAC formation (Cd59ab-/-) accelerates severe COVID-19. The degree of MAC but not C3 deposits in the lungs of C3-/-, C7-/- mice, and Cd59ab-/- mice as compared to their control mice is associated with the attenuation or acceleration of SARS-CoV-2-induced disease. Further, the lack of terminal complement activation for the formation of MAC in C7 deficient mice protects endothelial dysfunction, which is associated with the attenuation of diseases and pathologic changes. Our results demonstrated the causative effect of MAC in severe COVID-19 and indicate a potential avenue for modulating the complement system and MAC formation in the treatment of severe COVID-19.
Keywords: C3; Cd59; Complement; Endothelial dysfunction; MAC; Severe COVID-19.
© 2024. The Author(s).
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures





References
-
- Qin X, Gao B (2006) The complement system in liver diseases. Cell Mol Immunol 3(5):333–340 - PubMed
MeSH terms
Substances
Grants and funding
- R35 HL139930/HL/NHLBI NIH HHS/United States
- R01 DK129881/DK/NIDDK NIH HHS/United States
- R21 NR010361/NR/NINR NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
- HL139930/GF/NIH HHS/United States
- AHA962950/American Heart Association
- R01DK129881/GF/NIH HHS/United States
- R01 HL165265/HL/NHLBI NIH HHS/United States
- P51OD011104-62/GF/NIH HHS/United States
- 962950/AHA/American Heart Association-American Stroke Association/United States
- IK6BX005235/U.S. Department of Veterans Affairs
- IK6 BX005235/BX/BLRD VA/United States
- R01HL165265/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

