Vagus nerve stimulation recruits the central cholinergic system to enhance perceptual learning
- PMID: 39284963
- PMCID: PMC11932732
- DOI: 10.1038/s41593-024-01767-4
Vagus nerve stimulation recruits the central cholinergic system to enhance perceptual learning
Abstract
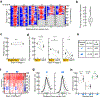
Perception can be refined by experience, up to certain limits. It is unclear whether perceptual limits are absolute or could be partially overcome via enhanced neuromodulation and/or plasticity. Recent studies suggest that peripheral nerve stimulation, specifically vagus nerve stimulation (VNS), can alter neural activity and augment experience-dependent plasticity, although little is known about central mechanisms recruited by VNS. Here we developed an auditory discrimination task for mice implanted with a VNS electrode. VNS applied during behavior gradually improved discrimination abilities beyond the level achieved by training alone. Two-photon imaging revealed VNS induced changes to auditory cortical responses and activated cortically projecting cholinergic axons. Anatomical and optogenetic experiments indicated that VNS can enhance task performance through activation of the central cholinergic system. These results highlight the importance of cholinergic modulation for the efficacy of VNS and may contribute to further refinement of VNS methodology for clinical conditions.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




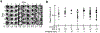











References
MeSH terms
Grants and funding
- T32 MH019524/MH/NIMH NIH HHS/United States
- n/a/National Science Foundation (NSF)
- R01 NS109885/NS/NINDS NIH HHS/United States
- R01 DC012557/DC/NIDCD NIH HHS/United States
- DC012557/U.S. Department of Health & Human Services | NIH | National Institute on Deafness and Other Communication Disorders (NIDCD)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

