Tonic and burst-like locus coeruleus stimulation distinctly shift network activity across the cortical hierarchy
- PMID: 39284964
- PMCID: PMC11537968
- DOI: 10.1038/s41593-024-01755-8
Tonic and burst-like locus coeruleus stimulation distinctly shift network activity across the cortical hierarchy
Abstract
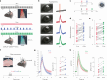
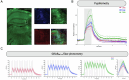
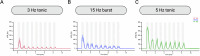
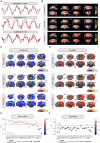
Noradrenaline (NA) release from the locus coeruleus (LC) changes activity and connectivity in neuronal networks across the brain, modulating multiple behavioral states. NA release is mediated by both tonic and burst-like LC activity. However, it is unknown whether the functional changes in target areas depend on these firing patterns. Using optogenetics, photometry, electrophysiology and functional magnetic resonance imaging in mice, we show that tonic and burst-like LC firing patterns elicit brain responses that hinge on their distinct NA release dynamics. During moderate tonic LC activation, NA release engages regions associated with associative processing, while burst-like stimulation biases the brain toward sensory processing. These activation patterns locally couple with increased astrocytic and inhibitory activity and change the brain's topological configuration in line with the hierarchical organization of the cerebral cortex. Together, these findings reveal how the LC-NA system achieves a nuanced regulation of global circuit operations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- PCEFP3_203005/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- PZ00P3_173984/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_172889/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- PZ00P3_208976/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- GNT1193857/Department of Health | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources

