Patient-reported outcomes during first-line palliative systemic therapy alternated with pressurized intraperitoneal aerosol chemotherapy for unresectable colorectal peritoneal metastases: a single-arm phase II trial (CRC-PIPAC-II)
- PMID: 39285036
- PMCID: PMC11525311
- DOI: 10.1007/s00464-024-11185-z
Patient-reported outcomes during first-line palliative systemic therapy alternated with pressurized intraperitoneal aerosol chemotherapy for unresectable colorectal peritoneal metastases: a single-arm phase II trial (CRC-PIPAC-II)
Abstract
Background: The CRC-PIPAC-II study prospectively assessed bidirectional therapy (BT) consisting of first-line palliative systemic therapy and electrostatic precipitation oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy (ePIPAC-OX) in patients with unresectable colorectal peritoneal metastases (CPM). This study describes the exploration of patient-reported outcomes (PROs).
Methods: In this phase II trial, 20 patients with isolated CPM were treated with up to three cycles of BT, each cycle consisting of two to three courses of systemic therapy, followed by ePIPAC-OX (92 mg/m2). Patients were asked to complete the EuroQoL EQ-5D-5L, EORTC QLQ-C30, and EORTC QLQ-CR29 questionnaires at baseline, during the first cycle of BT, and one and four weeks after each consecutive BT cycle. PRO scores were calculated and compared between baseline and each subsequent time point using linear-mixed modeling (LMM). PROs were categorized into symptom scales and function scales. Symptom scales ranged from 0 to 100, with 100 representing the maximum symptom load. Function scales ranged from 0 to 100, with 100 representing optimal functioning.
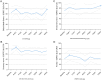
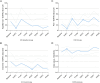
Results: Twenty patients underwent a total of 52 cycles of bidirectional therapy. Most PROs (29 of 37, 78%) were not significantly affected during trial treatment. In total, only eight PROs (22%) were significantly affected during trial treatment: Six PROs (index value, global health status, emotional functioning, C30, appetite, and insomnia) showed transient improvement at different time points. Two PROs transiently deteriorated: pain initially improved during the first BT cycle [- 16, p < 0.001] yet worsened temporarily one week after the first two BT cycles (+ 20, p < 0.001; + 17, p = 0.004; respectively). Abdominal pain worsened temporarily one week after the first BT cycle (+ 16, p = 0.004), before improving again four weeks after treatment ended (- 10, p = 0.004). All significant effects on Pros were clinically significant and all deteriorations in PROs were of temporary nature.
Discussion: Patients undergoing BT for unresectable CPM had significant, but reversible alterations in several PROs. Most affected PROs concerned improvements and only two PROs showed deteriorations. Both deteriorated PROs returned to baseline after trial treatment and were of a temporary nature. These outcomes help to design future studies on the role of ePIPAC in the palliative setting.
Keywords: Colorectal peritoneal metastases; Linear mixed modeling; PIPAC; Patient reported outcomes; Quality of life.
© 2024. The Author(s).
Conflict of interest statement
Vincent C.J. van de Vlasakker, Paulien Rauwerdink, Koen. P.B. Rovers, Emma C. Wassenaar, Geert-Jan Creemers, Maartje Los, Jacobus.W.A. Burger, Simon W. Nienhuijs, Onno Kranenburg, Marinus J. Wiezer, Robin J. Lurvink, Djamila Boerma, and Ignace H.J.T. de Hingh have no conflicts of interest or financial ties to disclose.
Figures
References
-
- Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT et al (2016) Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the analysis and research in cancers of the digestive system (ARCAD) database. Lancet Oncol 17(12):1709–1719 - PubMed
-
- Jacquet P, Sugarbaker PH (1996) Peritoneal-plasma barrier. Cancer Treat Res 82:53–64 - PubMed
-
- Speyer J (1985) The rationale behind intraperitoneal chemotherapy in gastrointestinal malignancies. Semin Oncol 12:23–28 - PubMed
-
- Dedrick RL, Flessner MF (1997) Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J Natl Cancer Inst 89(7):480–487 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

