Serotonin deficiency from constitutive SKN-1 activation drives pathogen apathy
- PMID: 39285192
- PMCID: PMC11405893
- DOI: 10.1038/s41467-024-52233-5
Serotonin deficiency from constitutive SKN-1 activation drives pathogen apathy
Abstract
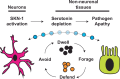
When an organism encounters a pathogen, the host innate immune system activates to defend against pathogen colonization and toxic xenobiotics produced. C. elegans employ multiple defense systems to ensure survival when exposed to Pseudomonas aeruginosa including activation of the cytoprotective transcription factor SKN-1/NRF2. Although wildtype C. elegans quickly learn to avoid pathogens, here we describe a peculiar apathy-like behavior towards PA14 in animals with constitutive activation of SKN-1, whereby animals choose not to leave and continue to feed on the pathogen even when a non-pathogenic and healthspan-promoting food option is available. Although lacking the urgency to escape the infectious environment, animals with constitutive SKN-1 activity are not oblivious to the presence of the pathogen and display the typical pathogen-induced intestinal distension and eventual demise. SKN-1 activation, specifically in neurons and intestinal tissues, orchestrates a unique transcriptional program which leads to defects in serotonin signaling that is required from both neurons and non-neuronal tissues. Serotonin depletion from SKN-1 activation limits pathogen defenses capacity, drives the pathogen-associated apathy behaviors and induces a synthetic sensitivity to selective serotonin reuptake inhibitors. Taken together, our work reveals interesting insights into how animals perceive environmental pathogens and subsequently alter behavior and cellular programs to promote survival.
© 2024. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures



Update of
-
Serotonin deficiency from constitutive SKN-1 activation drives pathogen apathy.bioRxiv [Preprint]. 2024 Feb 12:2024.02.10.579755. doi: 10.1101/2024.02.10.579755. bioRxiv. 2024. Update in: Nat Commun. 2024 Sep 16;15(1):8129. doi: 10.1038/s41467-024-52233-5. PMID: 38405962 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- T32 AG000037/AG/NIA NIH HHS/United States
- P40 OD010440/OD/NIH HHS/United States
- AG052374/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- T32 AG052374/AG/NIA NIH HHS/United States
- R01 AG058610/AG/NIA NIH HHS/United States
- AG077873/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30 AG068345/AG/NIA NIH HHS/United States
- AG000037/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- F31 AG077873/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

