Neural stem cells derived from α-synuclein-knockdown iPS cells alleviate Parkinson's disease
- PMID: 39285205
- PMCID: PMC11405526
- DOI: 10.1038/s41420-024-02176-z
Neural stem cells derived from α-synuclein-knockdown iPS cells alleviate Parkinson's disease
Abstract
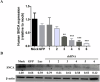
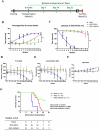
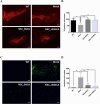
Stem cells have the potential to replace damaged or defective cells and assist in the development of treatments for neurodegenerative diseases, including Parkinson's disease (PD) and Alzheimer's disease. iPS cells derived from patient-specific somatic cells are not only ethically acceptable, but they also avoid complications relating to immune rejection. Currently, researchers are developing stem cell-based therapies for PD using induced pluripotent stem (iPS) cells. iPS cells can differentiate into cells from any of the three germ layers, including neural stem cells (NSCs). Transplantation of neural stem cells (NSCs) is an emerging therapy for treating neurological disorders by restoring neuronal function. Nevertheless, there are still challenges associated with the quality and source of neural stem cells. This issue can be addressed by genetically edited iPS cells. In this study, shRNA was used to knock down the expression of mutant α-synuclein (SNCA) in iPS cells that were generated from SNCA A53T transgenic mice, and these iPS cells were differentiated to NSCs. After injecting these NSCs into SNCA A53T mice, the therapeutic effects of these cells were evaluated. We found that the transplantation of neural stem cells produced from SNCA A53T iPS cells with knocking down SNCA not only improved SNCA A53T mice coordination abilities, balance abilities, and locomotor activities but also significantly prolonged their lifespans. The results of this study suggest an innovative therapeutic approach that combines stem cell therapy and gene therapy for the treatment of Parkinson's disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Yates D. Taking a closer look at PD pathology. Nat Rev Neurosci. 2019;20:511. - PubMed
-
- Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–60. - PubMed
-
- Pahwa R, Lyons KE. Levodopa-related wearing-off in Parkinson’s disease: identification and management. Curr Med Res Opin. 2009;25:841–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

