Liver-specific Coxsackievirus and adenovirus receptor deletion develop metabolic dysfunction-associated fatty liver disease
- PMID: 39285218
- PMCID: PMC11405401
- DOI: 10.1038/s41598-024-72561-2
Liver-specific Coxsackievirus and adenovirus receptor deletion develop metabolic dysfunction-associated fatty liver disease
Abstract
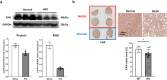
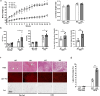
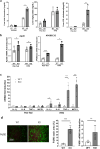
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a common liver disease associated with obesity and is caused by the accumulation of ectopic fat without alcohol consumption. Coxsackievirus and adenovirus receptor (CAR) are vital for cardiac myocyte-intercalated discs and endothelial cell-to-cell tight junctions. CAR has also been reported to be associated with obesity and high blood pressure. However, its function in the liver is still not well understood. The liver of obese mice exhibit elevated CAR mRNA and protein levels. Furthermore, in the liver of patients with non-alcoholic steatohepatitis, CAR is reduced in hepatocyte cell-cell junctions compared to normal levels. We generated liver-specific CAR knockout (KO) mice to investigate the role of CAR in the liver. Body and liver weights were not different between wild-type (WT) and KO mice fed a paired or high-fat diet (HFD). However, HFD induced significant liver damage and lipid accumulation in CAR KO mice compared with WT mice. Additionally, inflammatory cytokines transcription, hepatic permeability, and macrophage recruitment considerably increased in CAR KO mice. We identified a new interaction partner of CAR using a protein pull-down assay and mass spectrometry. Apolipoprotein B mRNA editing enzyme catalytic polypeptide-like 3C (APOBEC3C) demonstrated a complex relationship with CAR, and hepatic CAR expression tightly regulated its level. Moreover, Apolipoprotein B (ApoB) and Low-density lipoprotein receptor (LDLR) levels correlated with APOBEC3C expression in the liver of CAR KO mice, suggesting that CAR may regulate lipid accumulation by controlling APOBEC3C activity. In this study, we showed that hepatic CAR deficiency increased cell-to-cell permeability. In addition, CAR deletion significantly increased hepatic lipid accumulation by inducing ApoB and LDLR expression. Although the underlying mechanism is unclear, CARs may be a target for the development of novel therapies for MAFLD.
Keywords: APOBEC3C; Apolipoprotein B; Coxsackievirus and adenovirus receptor; Metabolic dysfunction–associated fatty liver disease; Paracellular permeability.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

